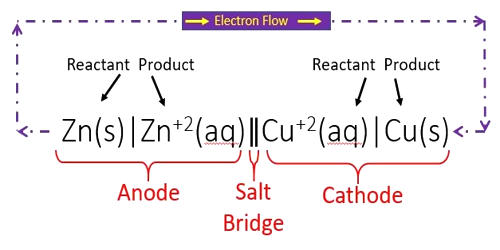
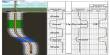
Cell notation or line notation is a shorthand description of voltaic or galvanic (spontaneous) cells. In chemistry, it is a shorthand way of expressing a certain reaction in an electrochemical cell. They are a shorthand description of voltaic or galvanic (spontaneous) cells. The cell anode and cathode (half-cells) are separated by two bars or slashes representing a salt bridge, with the anode on the left and cathode on the right. Individual solid, liquid, or aqueous phases within each half-cell are separated by a single bar. A positive cell potential indicates that the reaction proceeds spontaneously in the direction in which the reaction is written. Conversely, a reaction with a negative cell potential proceeds spontaneously in the reverse direction. Concentrations of dissolved species, in each phase written in parentheses and the state of each phase (usually s (solid), l (liquid), g (gas), or aq. (aqueous solution)) is included in a subscript after the species name. The description of the oxidation reaction is first, and the reduction reaction is last; when you read it, your eyes move in the direction of electron flow.
Some examples of this notation are: Zn°|Zn2+||Cl−|AgCl|Ag°
The reaction conditions (pressure, temperature, concentration, etc.), the anode, the cathode, and the electrode components are all described in this unique shorthand. This means that the left electrode (anode) is made of zinc, while the other one (right, cathode) is composed of a silver wire covered by a silver chloride layer which is not soluble. Both of the electrodes are immersed in aqueous media where zinc and chloride ions are present. Zn°|Zn2+, SO42−||SO42−, Cu2+|Cu°
The cell is represented by the rule that metals are written first and then the metal ions that are present in the electrolyte. This cell is very famous: the Daniell cell. If the electrodes are connected, a spontaneous reaction takes place. Zinc is oxidized, and copper ions are reduced. The anode half-cell is described first; the cathode half-cell follows. Within a given half-cell, the reactants are specified first and the products last.
Sometimes the state of each species into the cell is written. For example, in the zinc cell (shown above), we can write that zinc, silver, and silver chloride are solids, while zinc cation and chloride anion are in aqueous medium. The reaction conditions (pressure, temperature, concentration, etc.), the anode, the cathode, and the electrode components are all described in this unique shorthand. So, the new notation will be: Zn°s|Zn2+aq || Cl−aq|AgCls|Ag°s
The phase of each chemical (s, l, g, aq) is shown in parentheses. If the electrolytes in the cells are not at standard conditions, concentrations, and/or pressure, they are included in parentheses with the phase notation. It is possible to express the ion concentration too. For example, in the Galvanic cell: Zn°s|Zn2+aq (1 mol/L), SO42−aq (1 mol/L)||SO42−aq (1 mol/L)|Cu2+aq(1 mol/L)|Cu°s
In this case, all ions (sulfate, zinc, and copper) are in a concentration equal to 1 mol/L. To complete the electrical circuit, the zinc and copper(II) ion solutions are connected by a salt bridge (a supported ion solution).