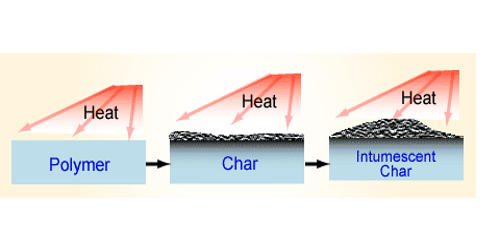
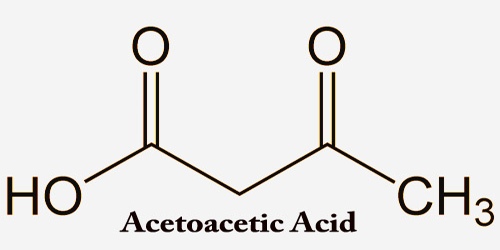
Charring is the incomplete combustion of organic material. It is a chemical process of incomplete combustion of certain solids when subjected to high heat. Charring of organic materials starts at temperatures considerably lower than that of soot formation. Heat distillation removes water vapor and volatile organic compounds (syngas) from the matrix. Burning of food during cooking (i.e., the production of nicely black toast) is an example of low-temperature charring. The residual black carbon material is char, as distinguished from the lighter colored ash. By the action of heat, charring removes hydrogen and oxygen from the solid, so that the remaining char is composed primarily of carbon.
At temperatures above about 300 °C, most of the organic materials undergo a slight thermal decomposition; hydrogen and other noncarbon elements are stripped from carbon chains and rings and the carbon condenses into a graphite-like structure. A combustion of a solid or liquid fuel takes place above the fuel surface in a region of vapors created by heating the fuel surface. The heat can come from the ambient conditions, from the presence of an ignition source, or from exposure to an existing fire. Polymers like thermoset, or most solid organic compounds like wood or biological tissue, exhibit charring behavior. The application of heat causes vapors or pyrolysis products to be released into the atmosphere where they can burn if in the proper mixture with air and if a competent ignition source is present.
Charring means partially burning so as to blacken the surface. In soldering with rosin flux, the temperature may get too high or the soldering time may take too long. In such cases, the flux loses its effect, especially because it burns and gets charred. There is no single chemical reaction taking place when wood burns. Wood is not even a chemical. It’s not a homogeneous material. It comes in different forms: damp, leafy, oak, teak, knotty (sappy) pine, etc.
Charring can result from naturally occurring processes like fire; it is also a deliberate and controlled reaction used in the manufacturing of certain products. The emergence of carbonaceous combustion products usually prevents flawless soldering. The mechanism of charring is part of the normal burning of certain solid fuels like wood. During normal combustion, the volatile compounds created by charring are consumed at the flames within the fire or released to the atmosphere, while combustion of char can be seen as glowing red coals or embers which burn without the presence of flames. This process is referred to as charring or chemical carbonization because charcoal is created from the burning of organic compounds.