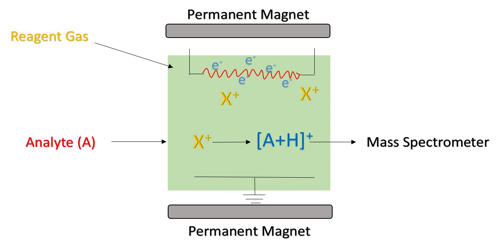
Chemical ionization (CI) is a soft ionization technique used in mass spectrometry. CI is based on the reactions of gaseous analyte molecules with an excess of ions formed by electron bombardment of a reagent gas. This was first introduced by Burnaby Munson and Frank H. Field in 1966. This technique is a branch of gaseous ion-molecule chemistry. It is another ionization technique used widely in GC MS applications. Reagent gas molecules are ionized by electron ionization, which subsequently reacts with analyte molecules in the gas phase in order to achieve ionization. CI relies on a reagent gas to assist in the ionization process of the compounds of interest. Negative chemical ionization (NCI), charge-exchange chemical ionization, and atmospheric-pressure chemical ionization (APCI) are some of the common variations of this technique. In chemical ionization mass spectroscopy, the sample molecule is first protonated in the gas phase using a very strong acid.
Chemical ionization uses a reagent gas to ionize sample molecules through ion-molecule reactions in the gas phase. CI has several important applications in identification, structure elucidation, and quantitation of organic compounds. CI is based on the reactions of gaseous analyte molecules with an excess of ions formed by electron bombardment of a reagent gas. Besides the applications in analytical chemistry, the usefulness in chemical ionization extends toward biochemical, biological, and medicinal fields as well. It uses a reagent gas to ionize sample molecules through ion-molecule reactions in the gas phase.
The earliest ionization technique was electron bombardment ionization (EI). The product obtained by EI has many fragments, which is difficult to analyze. CI, which produced very few fragments, began in the 1950s and has great potential in analytical chemistry. The sample molecules are then ionized by reagent ions through the molecular and ion reaction pathways. The 1970s was considered a milestone in the development of CI.
Applications
Chemical ionization or CI is another ionization technique used widely in GC MS applications. CI mass spectrometry is a useful tool in structure elucidation of organic compounds. The CI source is very similar to the EI source but the beam of electrons is used to create a plasma of ionized reagent gas. This is possible with CI because the formation of [M+1]+ eliminates a stable molecule, which can be used to guess the functional groups present. This process is less energetic than EI and generally produces less fragmentation. Besides that, CI facilitates the ability to detect the molecular ion peak, due to less extensive fragmentation. Chemical ionization can also be used to identify and quantify an analyte present in a sample, by coupling chromatographic separation techniques to CI such as gas chromatography (GC), high-performance liquid chromatography (HPLC) and capillary electrophoresis (CE). This allows selective ionization of an analyte from a mixture of compounds, where accurate and precise results can be obtained.