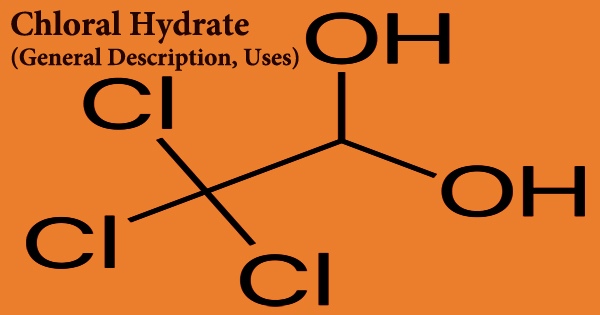
Chloral hydrate is a geminal diol with the formula C2H3Cl3O2 that may be separated as an analdehyde hydrate. The unfavorable dipole-dipole repulsion between the trichloromethyl carbon and the carbonyl carbon present in the parent carbonyl molecule is primarily responsible for this gem-diol’s relative stability. It’s a colorless substance. Its usage as a sedative and hypnotic prescription medication is restricted. It may also be used as a reagent and precursor in the laboratory.
It is made by adding one equivalent of water to chloral (trichloroacetaldehyde). In alkaline solutions, chloral hydrate is unstable, undergoing the last step of the haloform reaction to produce chloroform and formate ions. It produces the hemiacetal with ethanol in hydroalcoholic solutions. It’s debatable if this molecule is responsible for the well-known and potentially fatal action of ethanol plus chloral hydrate (the “MickeyFinn”).
Chloral hydrate was widely used for sedation in asylums and ordinary medical practice in the late 1800s, and it also became a popular drug of abuse. The synergy between two distinct CNS depressants might potentially have a role. Furthermore, ethanol promotes the reduction of chloral to the more active metabolite trichloroethanol by raising the concentration of nicotinamideadenine dinucleotide (NADH), while chloral can limit alcohol metabolism by inhibiting alcohol dehydrogenase.
Chloral hydrate is soluble in both water and ethanol, and it may easily be concentrated. To make a Mickey Finn, a solution of chloral hydrate in ethanol known as “knockout drops” was employed. It’s a weak acid since the CCl3 group has a high electron-withdrawing capacity. The pH of a 10% aqueous solution of chloral hydrate is 3.5 to 4.4, which is irritating to stomach mucous membranes. Chloral hydrate has a more respectable application as a chitin and fiber cleaning agent, as well as a major element in Hoyer’s mounting media, which is used to create permanent or semipermanent microscope slides of tiny organisms, histology sections, and chromosomal squashes.
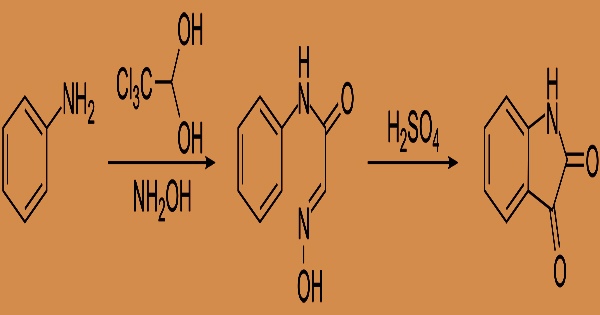
As a result, GI discomfort is frequent when the medication is taken undiluted or on an empty stomach. Chloral hydrate is now available as a pill, syrup, or suppository. Chloral hydrate might be difficult to come by due to its position as a controlled drug. As a result, other reagents have begun to replace chloral hydrate in microscopy operations. Ammoniacal silver nitrate is reduced by chloral hydrate. When mixed with an equivalent amount of menthol, camphor, or thymol, it liquefies.
Hoyer’s solution, a mounting media for microscopic viewing of many plant kinds such as bryophytes, ferns, seeds, and tiny arthropods, also contains chloral hydrate (especially mites). Despite the fact that chloral hydrate may operate as a hypnotic on its own, it is swiftly metabolized to trichloroethanol, which is thought to account for virtually all of the hypnotic action. Chloral hydrate is produced when chlorine and ethanol are combined in an acidic solution.
4 Cl2 + C2H5OH + H2O → Cl3CCH(OH)2 + 5 HCl
The haloform reaction occurs under basic circumstances, and chloral hydrate is hydrolyzed to form chloroform. Teratogenicity, reproductive consequences, and chronic toxicity of chloral hydrate have not been fully investigated. Likewise, no histological examinations have been carried out. Furthermore, chloral hydrate is employed as a reagent in organic solvents to deprotect acetals, dithioacetals, and tetrahydropyranyl ethers.
Chloral hydrate, also known as 2,2,2-trichloro-1,1-ethandiol (4.3.1), is produced by chlorinating ethanol or acetaldehyde and then adding water molecules to the resultant trichloroacetic aldehyde. Melzer’s reagent, an aqueous solution used to identify certain fungus species, contains chloral hydrate as a component. Trichloroethanol is converted to chloral and subsequently to trichloracetic acid, an inactive metabolite that is also extensively oxidized to acylglucuronides through conjugation with glucuronic acid.
In the past, chloral hydrate was given in gram doses. Long-term use of chloral hydrate is linked to a rapid build-up of tolerance to its effects, as well as potential addiction and side effects include rashes, stomach discomfort, and serious kidney, heart, and liver failure. The production of various chemical molecules begins with chloral hydrate. It is used to make chloral, which is made by distilling a combination of chloral hydrate and sulfuric acid, which acts as a desiccant.
One of the first synthetic medicines to treat insomnia was chloral hydrate. Bayer launched the medication phenobarbital under the trade name Luminal in 1912. Pentobarbital and secobarbital (also known by their original brand names Nembutal and Seconal) were developed in the 1930s. Chloral hydrate was still given, albeit barbiturates had essentially supplanted it as a sedative and hypnotic. Chloral hydrate becomes chloroform when it interacts with strong bases. Polymerization can occur as a result of contact with acids or exposure to light. Chloral hydrate is formed when chloral hydrate reacts with water. When it comes into contact with oxidizers, it can cause fire or explosions.
Information Sources: