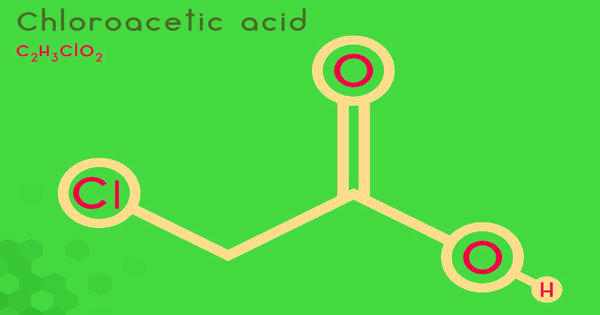
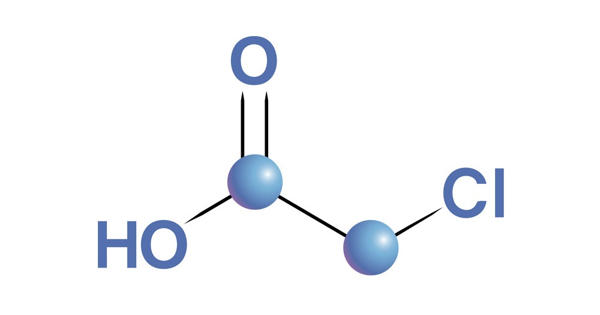
Chloroacetic acid is a chlorocarboxylic acid that is acetic acid carrying a 2-chloro substituent. It industrially known as monochloroacetic acid (MCA), is the organochlorine compound with the formula ClCH2CO2H. It has a role as an alkylating agent and a herbicide. This carboxylic acid is a useful building block in organic synthesis. It is a chlorocarboxylic acid and a haloacetic acid. It derives from acetic acid. It is a colorless solid. Related compounds are dichloroacetic acid and trichloroacetic acid.
Properties
Chloroacetic acid, solid is a colorless to light-brown crystalline material. Its solution is a colorless solution of the white crystalline solid. It is soluble in water and sinks in water. The acid concentration can be up to 80%.
Production
Chloroacetic acid was first prepared (in impure form) by the French chemist Félix LeBlanc (1813–1886) in 1843 by chlorinating acetic acid in the presence of sunlight, and in 1857 (in pure form) by the German chemist Reinhold Hoffmann (1831–1919) by refluxing glacial acetic acid in the presence of chlorine and sunlight, and then by the French chemist Charles Adolphe Wurtz by hydrolysis of chloroacetyl chloride (ClCH2COCl), also in 1857.
Chloroacetic acid is prepared industrially via two routes. The predominant method involves chlorination of acetic acid, with acetic anhydride as a catalyst:
H3C-COOH + Cl2 → ClH2C-COOH + HCl
ClH2C-COOH + NaOH → HO-CH2-COOH + NaCl
This route suffers from the production of dichloroacetic acid and trichloroacetic acid as impurities, which are difficult to separate by distillation:
H3C-COOH + 2 Cl2 → Cl2HC-COOH + 2 HCl
H3C-COOH + 3 Cl2 → Cl3C-COOH + 3 HCl
The second method entails hydrolysis of trichloroethylene:
ClHC=CCl2 + 3 H2O → HO-CH2-COOH + 3 HCl
The hydrolysis is conducted at 130–140 °C in a concentrated (at least 75%) solution of sulfuric acid. This method produces a highly pure product, unlike the halogenation route.
Uses
- In its largest-scale application, chloroacetic acid is used to prepare the thickening agent carboxymethyl cellulose and carboxymethyl starch.
- Chloroacetic acid is also used in the production of phenoxy herbicides by etherification with chlorophenols.
- Illustrative of its usefulness in organic chemistry is the O-alkylation of salicylaldehyde with chloroacetic acid, followed by decarboxylation of the resulting ether, producing benzofuran.
Health Hazards
It is transported as a molten liquid and therefore can cause thermal burns. It is toxic by ingestion, skin absorption, and inhalation of dust. It is corrosive to metals and tissue. It is toxic by inhalation, ingestion, and skin contact. It is used as an herbicide, preservative, and bacteriostat.
Information Source: