Chromatography is a method using mixed substances that depends on the speed at which they move through special media or chemical substances. It is a laboratory technique for the separation of a mixture. It consists of a stationary phase (a solid) and a mobile phase (a liquid or a gas). The mobile phase flows through the stationary phase. At its inception, it was used to separate plant pigments into their contributing chemicals.
Chromatography is much used in biochemistry and analytical chemistry. It is used in analysis, isolation, and purification, and it is commonly used in the chemical process industry as a component of small and large-scale production.
Flat-plane chromatography
A stationary phase is a flat plane, such as paper, or a substance on the glass.
Paper chromatography
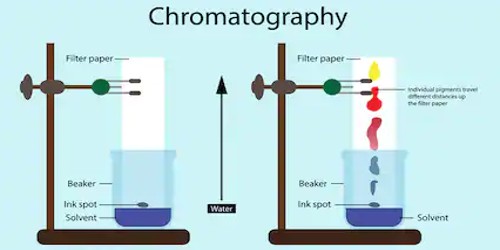
Paper chromatography is a technique for separating and identifying mixtures that are (or can be) colored. In paper chromatography, the stationary phase is a very uniform absorbent paper. It has been largely replaced by thin-layer chromatography but is still a powerful teaching tool. Double-way paper chromatography, also called ‘two-dimensional chromatography’, uses two solvents and rotates the paper 90° in between. It is useful for separating complex mixtures of compounds having similar polarity, for example, amino acids. The mobile phase is a suitable liquid solvent or mixture of solvents.
Thin-layer chromatography
Thin-layer chromatography (TLCC) is a common laboratory technique similar to paper chromatography. It is useful for separating mixtures of organic compounds. Instead of a stationary phase of paper, it uses a thin layer of adsorbent like silica gel, alumina, or cellulose on a flat substrate. Compared to paper, it has the advantage of faster runs, better separations, and the choice between different adsorbents. For even better resolution and to allow for quantification, high-performance TLC can be used.