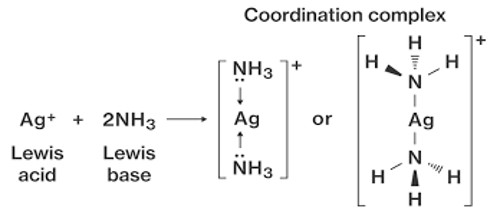
A coordination complex is an inorganic chemical compound where a central atom (usually a metal) has an electron shell which permits it to combine with one or more molecules. It is the product of a Lewis acid-base reaction in which neutral molecules or anions bond to a central metal atom by coordinate covalent bonds.
It is an ion with a central usually metallic atom or ion combined by coordinate bonds with a definite number of surrounding ions, groups, or molecules. These molecules, called ligands, have at least one pair of free electrons for binding. This type of bonding is different from a normal covalent bond in which each atom supplies one electron. The ions or molecules that bind to transition-metal ions to form these complexes are called ligands. A complex is a substance in which a metal atom or ion is associated with a group of neutral molecules or anions called ligands. The number of ligands bound to the transition metal ion is called the coordination number. Aluminum, tin, and lead, for example, form complexes such as the AlF63-, SnCl42- and PbI42-ions.
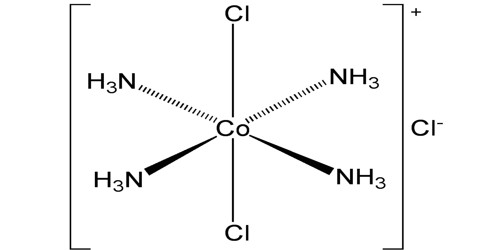
Alfred Werner developed a model of coordination complexes that explains the following observations. A complex ion has a metal ion at its center with a number of other molecules or ions surrounding it. At least three different cobalt(III) complexes can be isolated when CoCl2 is dissolved in aqueous ammonia and then oxidized by air to the +3 oxidation state. A fourth complex can be made by slightly different techniques. These complexes have different colors and different empirical formulas.
- CoCl3 6 NH3 —- orange-yellow.
- CoCl3 5 NH3 —- H2O red.
- CoCl3 5 NH3 —- purple.
- CoCl3 4 NH3 —- green.