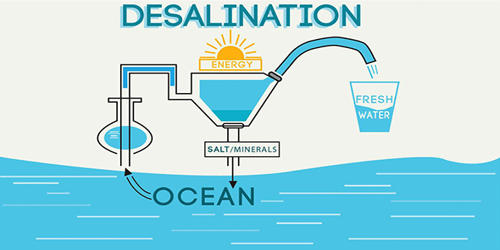
Desalination is a process that takes away mineral components from saline water. It is the process of removing salt from seawater. Desalination or desalting of water consists of a water treatment process by which sea or brackish water is converted into potable water for supplying communities that have the most difficulty accessing freshwater. More generally, desalination refers to the removal of salts and minerals from a target substance, as in soil desalination, which is an issue for agriculture.
Saltwater is desalinated to produce water suitable for human consumption or irrigation. There are different methods for minimizing salinity levels in the water, but Reverse Osmosis is the most extensive and advanced desalination system in the world, used in over 60% of facilities worldwide. The by-product of the desalination process is brine.
Water is very essential for all living beings. It covers nearly 70% of the earth’s surface. Even though the major portion of the earth is covered by water, there is a severe shortage of drinking water in most of the countries across the world. Safe drinking water is vital for all forms of life though it does not provide any calories. The desalination of seawater appears as a solution to this problem.
Methods of Desalination
At least three principle methods of desalination exist: thermal, electrical, and pressure.
- The oldest method, thermal distillation, has been around for thousands of years. In thermal distillation, the water is boiled and then the steam is collected, leaving the salt behind.
- A second major type of desalination utilizes electric current to separate the water and salt. Typically, electric current will be used to drive ions across a selectively permeable membrane, carrying the dissociated salt ions with it.
- A third principle method of desalination is reverse osmosis, in which pressure is used to drive water through a selectively permeable membrane, leaving the salt behind.
Desalination is used on many seagoing ships and submarines. It is a water treatment process that turns salt water into fresh water. Most of the modern interest in desalination is focused on the cost-effective provision of fresh water for human use. Along with recycled wastewater, it is one of the few rainfall-independent water sources. It is the process of removing dissolved minerals from the water so that people can use the treated water for applications that usually require fresh water. This is the most extensive and advanced desalination system in the world, used in over 60% of facilities worldwide. The reverse osmosis process consists of applying pressure to a saltwater solution and forcing it through a semi-permeable membrane whose function is to allow the passage of the solvent (water) but not the solute (dissolved salts).
Desalinated water can be used for agriculture, industry, and domestic use. Due to its energy consumption, desalinating seawater is generally more costly than fresh water from rivers or groundwater, water recycling, and water conservation. However, these alternatives are not always available and depletion of reserves is a critical problem worldwide. When large quantities of fresh water are needed, desalination might not be as cost-effective as using fresh water sources because the process is very energy-intensive. Desalination processes are usually driven by either thermal (in the case of distillation) or electrical (in the case of reverse osmosis) as the primary energy types.