Diatomic Bromine is a molecule formed when two bromine atoms combine together. It is a dark reddish-brown fuming liquid with a pungent odor. Bromine (Br2) is a red-brown liquid at ordinary temperature. It is very volatile. It is denser than water and soluble in water. Bromine was discovered by French chemist Antoine J.Balard in 1826.
It gives very dense red-brown vapours that are highly toxic to mucous membranes. It is toxic by inhalation. It accelerates the burning of combustible material. It is very corrosive to tissue and to metals.
Properties
Bromine has various physical properties. It has a red-brown color and is a dense liquid having a melting point and boiling point of -7° Celsius and 58.9° Celsius, respectively. It is heavy and nonmetallic. Bromine evaporates quickly at room temperature due to its liquid state. It exhibits an unpleasant odor, which is three times as dense as water.
- Density: 3.1023 at 77 ° F
- Molecular Weight/ Molar Mass: 159.808 g/mol
- Boiling Point: 139.2 ° F at 760 mm Hg
- Melting Point: 19 ° F
- Chemical Formula: Br-Br
- Appearance: Red to amber coloured gas
- Solubility in water: 0.33 mg/ mL.
Bromine reacts with sodium carbonate forms sodium bromide, sodium bromate and carbon dioxide.
3Br2 + 3Na2CO3 → 5NaBr + NaBrO3 + 3CO2
Bromine dissolves in water forming hydrogen bromide and Hypobromous acid. The chemical equation is given below.
Br2 + H2O → HBr + HBrO
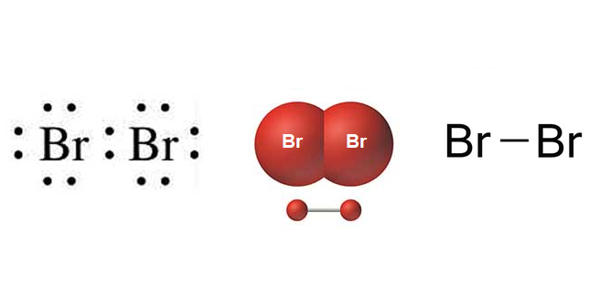
Uses
- Bromine can be used in oil and gas well drillings and in gold mining extraction processes.
- It is used in gold mining extraction processes and in oil- and gas-well drilling.
- Compounds with 32% bromine are used in textile coatings, spray-bonded nonwovens, adhesives, and fibres.
- Used in the manufacture of flame-retardant materials used most commonly in markets.
- It is also used for analytical processes and for the production of organic compounds.
- Bromine is used mostly for the production of organic compounds and for analytical purposes.
- Bromine is purified from the salts taken from rocks and seawater. It is sold as salts or other compounds because pure bromine is expensive and difficult to produce.
- It is used as an emulsifier in various citrus-flavored soft drinks.
Health Effects
Bromine is highly corrosive to human tissue in a liquid state, and the vapors irritate the eyes and throat. Bromine vapors become extremely toxic with inhalation. Humans can absorb the organic bromine through the skin, during breathing, and with food. The most important health problems that can be caused by organic bromine-containing contaminants are nervous system malfunctions and genetic material abnormalities.
Information Source: