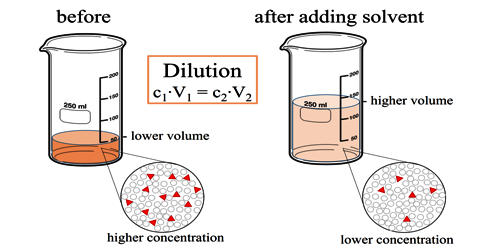
Dilution is the action of making a liquid more dilute. It is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding more water to a solution. To dilute a solution means to add more solvent without the addition of more solute. It is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. The resulting solution is thoroughly mixed so as to ensure that all parts of the solution are identical. Dilutions can be performed in the laboratory with various tools, depending on the volumes required and the desired accuracy.
Dilution can also be achieved by mixing a solution of higher concentration with an identical solution of lesser concentration. The same direct relationship applies to gases and vapors diluted in the air for example. Diluting solutions is a necessary process in the laboratory, as stock solutions are often purchased and stored in very concentrated forms. Although, thorough mixing of gases and vapors may not be as easily accomplished. For the solutions to be usable in the lab (for a titration, they must be accurately diluted to a known, lesser concentration. A common dilution problem involves deciding how much of a highly concentrated solution is requires to make the desired quantity of a solution of lesser concentration. The highly concentrated solution is typically referred to as the stock solution.
It can be defined as the mass of the original substrate or previously deposited track melted, divided by the sum of the combined mass of substrate and added material melted. For example, if there are 10 grams of salt (the solute) dissolved in 1 liter of water (the solvent), this solution has a certain salt concentration (molarity). If one adds 1 liter of water to this solution the salt concentration is reduced. The diluted solution still contains 10 grams of salt (0.171 moles of NaCl).
Mathematically this relationship can be shown by equation:
c1 × V1 = c2 × V2
where,
- c1 = initial concentration or molarity
- V1 = initial volume
- c2 = final concentration or molarity
- V2 = final volume.