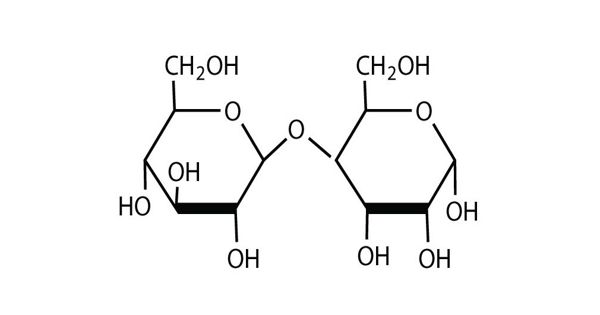
A disaccharide is a sugar (a carbohydrate) composed of two monosaccharides, such as glucose and fructose that make up the disaccharide sucrose. It is a substance that is composed of two molecules of simple sugars (monosaccharides) linked to each other. Three common disaccharides are sucrose, maltose, and lactose.
It is formed when two sugars are joined together and a molecule of water is removed. These are formed through dehydration reactions in which a total of one water molecule is removed from the two monosaccharides. It is the sugar formed when two monosaccharides are joined by glycosidic linkage. They are composed of two monosaccharide units linked together by a glycosidic bond. The most common glycosidic bonds connecting monosaccharide units are O-glycosidic bonds in which the oxygen from a hydroxyl group becomes linked to the carbonyl carbon. Due to this, disaccharides cannot hydrolyze, meaning their molecules are unable to be broken down through a reaction with water.
Disaccharides are those carbohydrates that on hydrolysis with acids or enzymes give two molecules of monosaccharides which can either be the same or different. These are made up of two monosaccharides and are commonly found in fruits and vegetables, including sugar beets and sugar cane, and as the lactose in dairy products. For example, milk sugar (lactose) is made from glucose and galactose whereas cane sugar (sucrose) is made from glucose and fructose. The reducing disaccharides of pharmaceutical importance are maltose, cellobiose, lactose, gentiobiose, and rutinose.
Disaccharides are formed by the elimination of a water molecule between two monosaccharides (different or identical) with the formation of an ether bond. The oxide linkage is formed after the loss of the water molecule and then the two monosaccharides are formed by that linkage. They are one of the four chemical groupings of carbohydrates (monosaccharides, disaccharides, oligosaccharides, and polysaccharides). The most common types of disaccharides—sucrose, lactose, and maltose—have 12 carbon atoms, with the general formula C12H22O11. The differences in these disaccharides are due to atomic arrangements within the molecule.
Functions
Disaccharides are carbohydrates found in many foods and are often added as sweeteners. In your body, a disaccharide function is to provide your body with a quick source of energy. Sucrose, for example, is table sugar, and it is the most common disaccharide that humans eat. It is also found in other foods like beetroot. Because they’re only made up of two sugar molecules, they’re easily broken down by enzymes in your digestive system into their respective monosaccharides and then absorbed into your bloodstream.
When disaccharides like sucrose are digested, they are broken down into their simple sugars and used for energy. Lactose is found in breast milk and provides nutrition for infants. Maltose is a sweetener that is often found in chocolates and other candies.
Information Source: