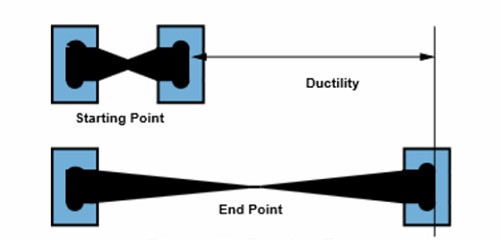
Ductility is when a solid material stretches under tensile stress. It is a measure of a metal’s ability to withstand tensile stress—any force that pulls the two ends of an object away from each other. If ductile, a material may be stretched into a wire. It means that a metal can be changed to another form by pulling, compression or twisting. It is the property of a substance by which it can be drawn into thin sheets. Malleability, a similar property, is a material’s ability to deform under pressure (compressive stress). It is property of a material to be drawn into thin wires. If malleable, a material may be flattened by hammering or rolling.
Both of these properties are aspects of plasticity. Plasticity is how far a solid material can be deformed without fracture. Metals having ductile property can be stretched into wires. An example is copper wire. These properties are commonplace in metals and are dependent on temperature and pressure. It also refers to the ability of a metal to change its form under tensile stress. This was investigated by Percy Williams Bridgman as part of his 1946 Nobel Prize-winning work on high pressures.
Ductility and malleability do not always go together. A metal’s ductility is measured by looking at its tensile strength. The bend test is the commonly used test for determining the ductility of a metal. Gold has high ductility and malleability, but lead has low ductility and high malleability. The word ductility is sometimes used to embrace both types of plasticity. It is the ability of a material to be drawn or plastically deformed without fracture.
Examples of Ductile metals are Aluminium, Copper, Gold, etc. Gold, copper, aluminum, and steel have high ductility. Examples of metals that are not very ductile include tungsten and high-carbon steel. Nonmetals are not generally ductile.