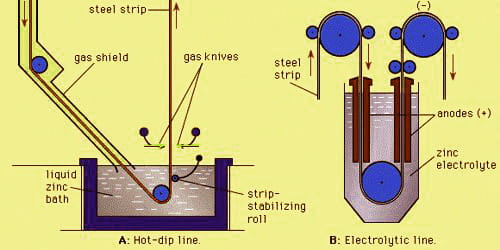
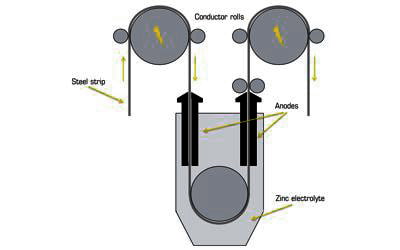
Electrogalvanizing is a process in which a layer of zinc is bonded to steel in order to protect against corrosion. In this process, a zinc layer is applied using an electric current. A zinc electrolyte bath equipped with two electrodes is utilized – an anode (positive pole) and the steel parts to be galvanized as a cathode (negative pole), to which current is applied. It is an electroplating technique used to place a layer of zinc metal on top of a steel surface.
Electrogalvanizing is one of the most commonly used processes in metal finishing and is also the most cost-effective technique for ensuring consistent protection against corrosion.
The process involves electroplating, running a current of electricity through a saline/zinc solution with a zinc anode and steel conductor. Dissolved zinc is also included in the bath, and is introduced as a concentrate through metering pumps, and a conducting salt, typically caustic soda (sodium hydroxide/NaOH) is also added to boost conductivity. Zinc electroplating maintains a dominant position among other electroplating process options, based upon electroplated tonnage per annum. The steel components are cleaned and prepared for the coating process with the help of pickling baths and upstream degreasing.
According to the International Zinc Association, more than 5 million tons are used yearly for both hot-dip galvanizing and electroplating. Since process work is performed with a cyanide-free and eco-friendly zinc bath, it must be ensured that the components to be coated are free of impurities, like surface scale and rust. The plating of zinc was developed at the beginning of the 20th century. At that time, the electrolyte was cyanide-based.
A significant innovation occurred in the 1960s, with the introduction of the first acid chloride-based electrolyte. The 1980s saw a return to alkaline electrolytes, only this time, without the use of cyanide. To obtain a highly reliable product quality, each step of the process should be closely observed and performed within the specified limits. It involves immersing a steel component into a solution containing zinc salts followed by the application of electricity to induce an electrochemical reaction on top of the steel.
Therefore, the electrogalvanization process includes four active baths as well as complimentary cleaning and rinsing baths. In order to realize excellent product quality, it is important to ensure that the composition of the numerous baths remains within the given range. The most commonly used electrogalvanized cold-rolled steel is SECC steel. Compared to hot-dip galvanizing, electroplated zinc offers these significant advantages:
- Lower thickness deposits to achieve comparable performance
- Broader conversion coating availability for increased performance and color options
- Brighter, more aesthetically appealing, deposits.
Information Source: