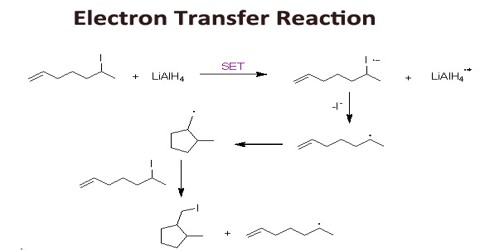
Electron Transfer Reaction
Electron transfer reactions are reactions of the oxidative-reductive type. They are of great importance in nature, but also in technology, and have been in the center of interest during the last 50 years, which is evident from several thousands of publications in the field. Most of the reactions by which anion radicals and cation radicals are formed are electron transfer (ET) reactions. Since electron transfer is the simplest of all chemical reactions, its study is especially fundamental, and we might expect to find some extremely fast reactions in this domain. First, let’s look at three different methods of effecting electron transfer in the organic laboratory.
Electron Transfer Reaction constitutes one of the broadest and most important classes of reactions in chemistry. All reactions that involve molecular oxygen, such as combustion and corrosion, are electron transfer reactions. Biological processes, such as respiration, photosynthesis, and the breakdown of food molecules, consist of sequences of electron transfer reactions that serve to transport and utilize energy from the sun. Batteries are devices that allow us to utilize the free energy of electron transfer reactions.
In an electron transfer reaction, an element undergoing oxidation loses electrons, whereas an element gaining electrons undergoes reduction. In the aluminum‐oxygen example, the aluminum was oxidized, and the oxygen was reduced because every electron transfer reaction involves simultaneous oxidation and reduction. These reactions are frequently called redox reactions.
About Electron Transfer
Electron transfer, or the act of moving an electron from one place to another, is amongst the simplest of chemical processes, yet certainly one of the most critical. Without stringent control of electrons in living organisms, life could simply not exist. Transition metals such as copper and iron play leading roles in electron transport as one-electron redox-active centers within proteins that are used to effectively move electrons around.
There are several classes of electron transfer, they are:
Inner-Sphere Electron Transfer
Outer-Sphere Electron Transfer –
Five steps of an outer sphere reaction
- reactants diffuse together out of their solvent shells => precursor complex (requires work =wr)
- changing bond lengths, reorganize solvent => activated complex
- Electron transfer
- Relaxation of bond lengths, solvent molecules => successor complex
- Diffusion of products (requires work=wp)
Heterogeneous Electron Transfer
The first generally accepted theory of ET was developed by Rudolph A. Marcus to address outer-sphere electron transfer and was based on a transition-state theory approach. The Marcus theory of electron transfer was then extended to include inner-sphere electron transfer by Noel Hush and Marcus. The resultant theory, called Marcus-Hush theory, has guided most discussions of electron transfer ever since.
Methods of Electron Transfer Reactions
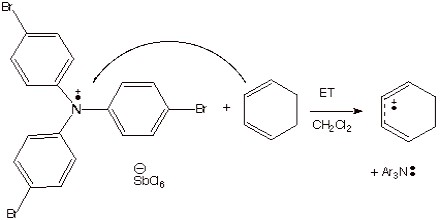
Chemical Methods. As an example of the formation of anion radicals by chemical methods, we have already considered the transfer of an electron from sodium or potassium metal to a neutral substrate, such as benzophenone (to form the ketyl). A chemical method for forming cation radicals which was emphasized was the use of a stable aminium salt to ionize an electron from an organic substrate.
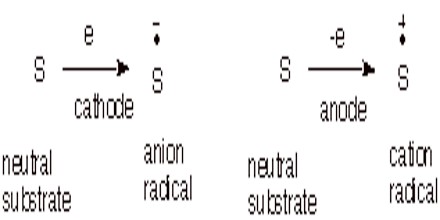
Electrochemical Methods. We can also form anion and cation radicals by electrochemical reduction or oxidation at an appropriate electrode at an appropriate reduction or oxidation potential.
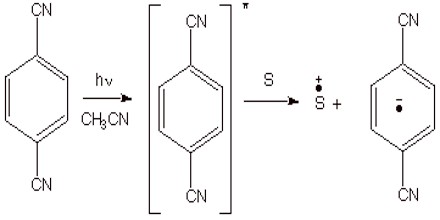
Photochemical Methods. Besides the chemical and electrochemical methods of generation, there are also some very efficient photochemical methods. The most common approach is to use a photosensitizer to absorb uv light, and to allow the resulting excited state of the photosensitizer to remove an electron to an organic substrate (or to transfer an electron to the organic substrate). This is illustrated for the case of 1,4-dicyanobenzene as the photosensitizer. If, for example, 1,3-cyclohexadiene is the substrate, this results in a cation radical chain process which forms the previously mentioned Diels-Alder dimers.