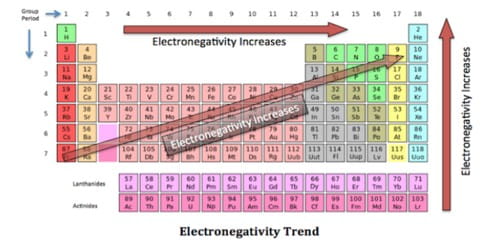
Electronegativity refers to the ability of an atom to attract shared electrons in a covalent bond. Electronegativity, symbol χ, is a chemical property that says how well an atom can attract electrons towards itself. It is a measure of the tendency of an atom to attract a bonding pair of electrons. The electronegativity of an atom is influenced by the atom’s atomic number and the distance between the atom’s valence electrons (the outermost electrons that take part in chemical bonding) and its nucleus. An atom with high electronegativity attracts electrons strongly, while an atom with low electronegativity attracts them weakly.
It was first theorized by Linus Pauling in 1932 as part of his valence bond theory and is related to other chemical properties. Generally, electronegativity increases from the bottom-left to the upper-right of the periodic table; this is known as a periodic trend. The most electronegative element is fluorine, followed by oxygen, chlorine, and nitrogen.
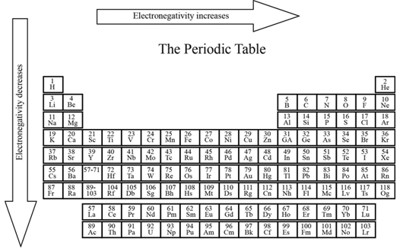
There are many ways to calculate the electronegativity of an atom. The most common way of calculation is the one suggested by Linus Pauling, and it gives the relative Pauling scale. This scale gives elements dimensionless quantities (values) between 0.7 to 3.98, with hydrogen being at 2.20. The higher the value of the electronegativity, the more strongly that element attracts the shared electrons. If atoms bonded together have the same electronegativity, the shared electrons will be equally shared.
The opposite of electronegativity is electropositivity; the measure of how well an atom gives away electrons. It varies in a predictable way across the periodic table.