Electroplating is the process of applying a metal coating on another piece of metal (or another conductive surface) through an electro-deposition process. It is a general name for processes that create a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. In electroplating, the deposited metal becomes part of the existing product with the plating/coating. This process can be used to give objects increased wear resistance, corrosion protection, or aesthetic appeal, as well as increased thickness.
Electroplating is the process of coating with metal by means of an electric current. The process uses an electric current to reduce dissolved metal cations to develop a lean coherent metal coating on the electrode.
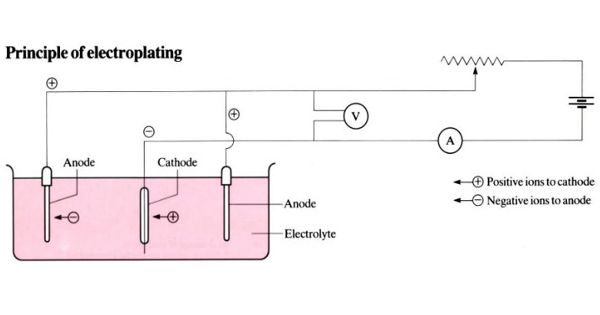
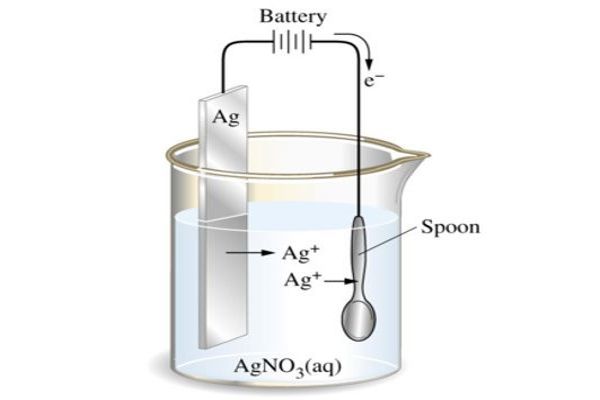
The part to be coated acts as the cathode (negative electrode) of an electrolytic cell; the electrolyte is a solution of a salt of the metal to be coated, and the anode (positive electrode) is usually either a block of that metal or of some inert conductive material. In Electroplating, both an anode and a cathode (the metal part to be coated) are immersed in an electrolytic bath that is composed of a solution of salts, including the metal to be plated. The current is provided by an external power supply. A direct current (DC) of electricity is passed through the solution, affecting the transfer of metal ions onto the cathodic surface, plating the metal onto the item. After electroforming, it is possible to perform electroplating to add a coating to improve corrosion-resistance or to get a more attractive (cosmetic) product.
Here are the most commonly used materials: Nickel, Black nickel/chromium, Chromium, Palladium, Gold, Silver, Copper, Tin, Platinum, Ruthenium, Cadmium, Brass, Zinc, etc.
Electroplating comes with several material capabilities. Electroplating is widely used in industry and decorative arts to improve the surface qualities of objects—such as resistance to abrasion and corrosion, lubricity, reflectivity, electrical conductivity, or appearance. The materials used in the plating (coating) process depend on the composition of the plating bath and the deposition conditions.
Electroplating is a popular metal finishing and improving process used in a wide range of industries for various applications. It may also be used to build up thickness on undersized or worn-out parts or to manufacture metal plates with complex shapes, a process called electroforming. It is primarily used to change the physical properties of an object. It is also used to purify metals such as copper.
Information Source: