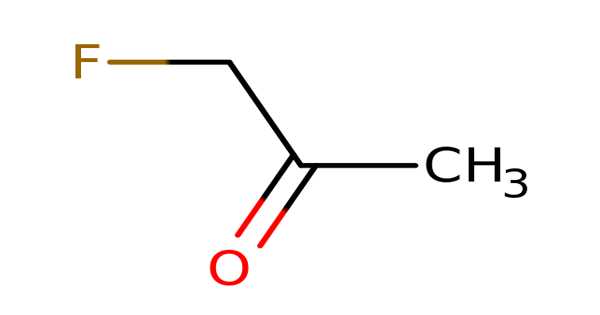
Fluoroacetone has the chemical formula C3H5FO and is an organofluorine compound. The compound, unlike trifluoroacetone, only has one fluorine atom. The substance is a colorless to light yellow liquid under normal conditions. Fluoroacetone is a highly toxic and flammable compound as well. Fluoroacetone fumes can combine with air to form an explosive mixture.
Fluoroacetone was synthesized by dibrominating monochloroacetone and then exchanging one bromine atom for fluorine in an exchange reaction with mercuric fluoride under strictly controlled conditions.
Properties
- Boiling point: 75 °C (lit.)
- Density: 1.054 g/mL at 25 °C (lit.)
- Flash point: 45 °F
- Storage temp.: 2-8°C
- Form: Powder
- Color: Red to dark red
- Specific Gravity: 1.054
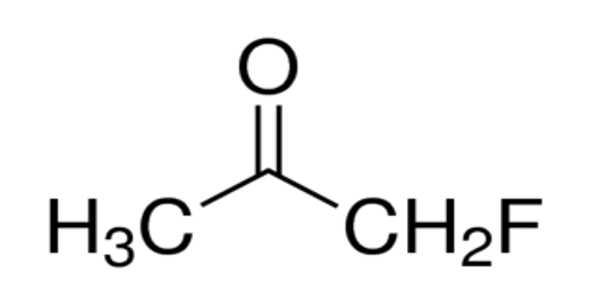
Synthesis
Fluoroacetone can be obtained by a reaction of triethylamine tri-hydro fluoride with bromoacetone.
Fluoroacetones are synthesized by dehydrohalogenation hydrogen halide-fluoroacetone complexes with sulphur trioxide at temperatures above 0 DEG C. The complexes may be obtained as byproducts of the production of fluoroacetones from hexachloroacetone and hydrogen fluoride and include alcohols and ethers such as hexafluoro-2 – chloro – isopropanol, heptafluoroisopropanol, 1-chloro-hexafluoroisopropanol, and 2-(1 – chlorohexafluoro Dehydrohalogenation can take place in either the vapour or liquid phase. Distillation separates the resulting ketones from the byproduct halosulphonic acids.
Applications
The kinetics of the ketone-catalyzed decomposition of peroxymonosulfuric acid (Caro’s acid) are studied using fluoroacetone as a catalyst. It is also used as a starting material for the synthesis of higher fluoroketones.
In contrast to other halogenated acetone derivatives such as bromoacetone or chloroacetone, fluoroacetone has not been used as a lachrymatory substance.
Safety
- Fatal if swallowed.
- Fatal in contact with skin.
- Fatal if inhaled.