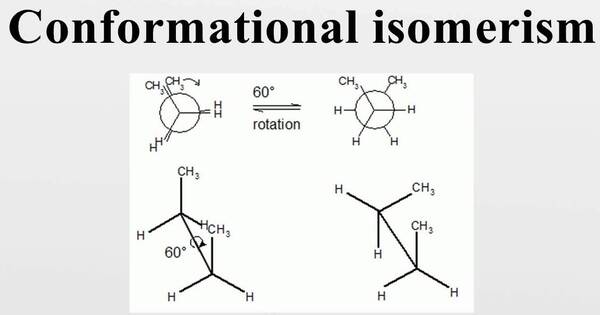
Conformational isomerism is the phenomenon in which molecules can exist in distinct spatial configurations due to rotation around single bonds. In chemistry, it is a type of stereoisomerism in which the isomers can be interconverted simply by rotating about formally single bonds (see figure on single bond rotation). This type of isomerism results from the free rotation of single bonds, which allows the molecule to take on different three-dimensional configurations while maintaining the same atomic connectivity.
While any two arrangements of atoms in a molecule that differ by rotation about single bonds might be considered separate conformations, conformations that correspond to local minima on the potential energy surface are known as conformational isomers or conformers. The different spatial arrangements are called conformations, and they are interconvertible by rotation about the single bonds.
Conformations that correspond to local maxima on the energy surface are transitional states between conformational isomers with local minima. Rotations about single bonds need to overcome a rotational energy barrier in order to interconvert one conformer into another. If the energy barrier is low, there is free rotation and a sample of the compound exists as a rapidly equilibrating mixture of multiple conformers; if the energy barrier is high enough, there is restricted rotation, and a molecule may exist for a relatively long time as a stable rotational isomer or rotamer (an isomer resulting from hindered single-bond rotation).
Conformational isomerism is especially common in molecules with flexible structures, including alkanes and cycloalkanes. Rotation about single bonds permits these molecules to transition between distinct conformations. When the time scale for interconversion is long enough to isolate individual rotamers (often defined as a half-life of interconversion of 1000 seconds or longer), the isomers are referred to as atropisomers. The ring-flipping of substituted cyclohexanes is another typical type of conformational isomerism.
Example
The most common example of conformational isomerism is found in ethane (C2H6). Ethane consists of two carbon atoms bonded to each other with single bonds and each carbon atom bonded to three hydrogen atoms. The rotation around the carbon-carbon bond allows for different spatial arrangements of the hydrogen atoms relative to each other.
The staggered conformation of ethane is the most stable, as it minimizes steric hindrance by separating the hydrogen atoms on each carbon atom as much as possible. The eclipsed conformation, in which hydrogen atoms on consecutive carbon atoms are immediately opposite one another, is less stable due to higher steric hindrance.
Conformational analysis is critical for understanding the behavior of molecules in various chemical reactions and biological processes since different conformations might have varied reactivities, energy, and physical characteristics.