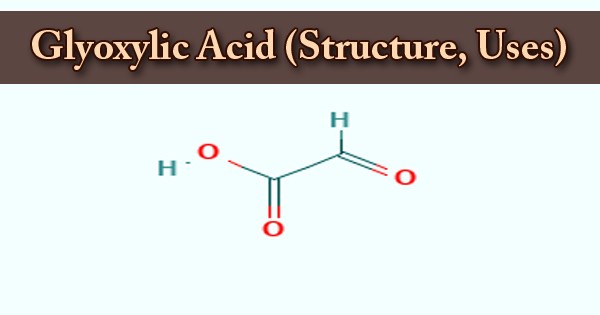
A 2-oxo monocarboxylic acid that is an acetic acid bearing an oxo group at the alpha carbon atom is glyoxylic acid or oxoacetic acid. It is an organic compound; it is also a naturally occurring colorless solid that is industrially useful. A human metabolite, an Escherichia coli metabolite, a Saccharomyces cerevisiae metabolite, and a mouse metabolite play a part in glyoxylic acid. It is a 2-oxo monocarboxylic acid, an aldehydrous acid, and a glyoxylate conjugate acid. This compound is used in the following products: cosmetics and products for personal care. Other releases of this material are likely to occur from the environment: indoor use as a processing aid.
Hydrogen ions are donated by carboxylic acids if a base is present to accommodate them. They react with all bases, both organic (amines, for example) and inorganic, in this way. Their baseline reactions referred to as “neutralization” are followed by the production of large quantities of heat. Although the structure of glyoxylic acid is defined as having a functional group of aldehydes, in some circumstances the aldehyde is only a minor component of the type most prevalent. Instead, it also occurs as a cyclic dimer or a hydrate.
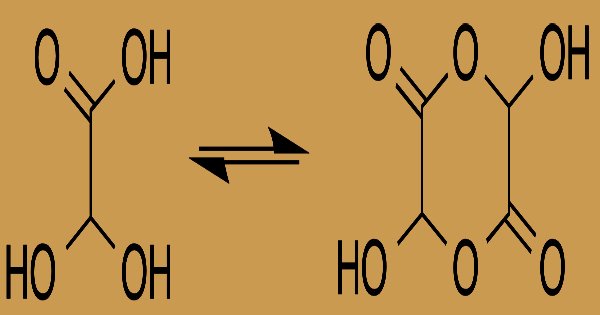
Crystals from water; melting point: 70-75 °C; obnoxious odor; strong corrosive acid; K= 4.6X10-4; deliquesces; except for some stainless steel alloys, most stable metals attack; aq soln appears to develop a yellow tint. The equilibrium constant (K) for dihydroxyacetic acid formation at room temperature is 300:

In solution, in equilibrium with a hemiacetal dimer shape, the monohydrate exists:
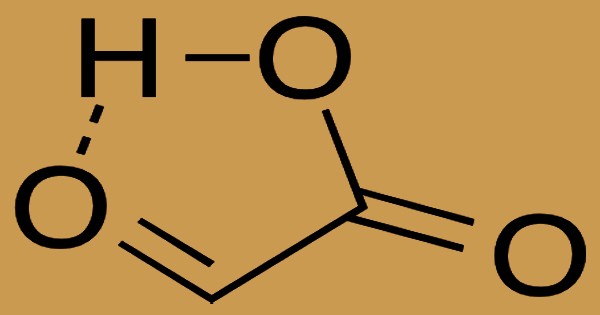
Water plus salt is created by neutralization between an acid and a base. Carboxylic acids containing six or less atoms of carbon are free or moderately soluble in water, whereas those containing more than six atoms of carbon are partially soluble in water. In order to yield hydrogen ions, soluble carboxylic acid dissociates to a degree in water. In isolation, the composition of the aldehyde has a cyclic hydrogen-bonded structure with the aldehyde carbonyl in near proximity to the carboxyl hydrogen as a significant conformer:
In an aqueous solution, carboxylic acids and liquid or molten carboxylic acids can react to form gaseous hydrogen and metal salt with active metals. In general, such reactions also occur for solid carboxylic acids but are sluggish if the solid acid remains dry. Glyoxal can be oxidized to glyoxylic by hot nitric acid, but this reaction is extremely exothermic and vulnerable to thermal runaway. In addition, the primary side product is oxalic acid; ozonolysis of maleic acid is also successful. And “insoluble” carboxylic acids can absorb sufficient water from the air and dissolve enough in glyoxylic acid to corrode or dissolve sections and containers of iron, steel, and aluminum.
Glyoxylate is a glyoxylate cycle intermediate that allows fatty acids to be converted into carbohydrates by animals, such as bacteria, fungi, and plants. Via isocitrate lyase operation, which transforms isocitrate into glyoxylate and succinate, the glyoxylate cycle is initiated. For dry, strong carboxylic acids, the reaction is slower. To induce the release of gaseous hydrogen cyanide, insoluble carboxylic acids react with cyanide solutions. Reactions of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides produce flammable and/or toxic gases and heat.
Glyoxylic acid is typically subjected to an electrophilic aromatic replacement reaction with phenols, a versatile step in the synthesis of many other compounds. Contact can cause serious eye and skin burns; eye and skin irritation can be caused by vapor exposure. As a net formulation process, the sequence of reactions in which glyoxylic acid reacts with guaiacol, the phenolic component followed by oxidation and decarboxylation, provides a path to vanillin. A part of the Hopkins-Cole reaction is glyoxylic acid, used to search for the presence of tryptophan in proteins.
Information Sources: