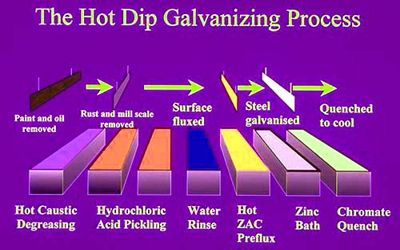
Hot-dip galvanizing is a process developed to prevent steel from corroding. Hot-dip galvanization is a form of galvanization. It is the process of coating iron and steel with zinc, which alloys with the surface of the base metal when immersing the metal in a bath of molten zinc at a temperature of around 449°C (840°F). Before the process can take place, the steel goes through a thorough chemical clean which removes all rust, oil, and mill scale from the surface.
Hot-dip galvanizing is the process of coating fabricated steel by immersing it in a bath of molten zinc. It provides a number of benefits to the steel it protects.
When exposed to the atmosphere, the pure zinc (Zn) reacts with oxygen (O2) to form zinc oxide (ZnO), which further reacts with carbon dioxide (CO2) to form zinc carbonate (ZnCO3), a usually dull grey, fairly strong material that protects the steel underneath from further corrosion in many circumstances. When the cooling process is complete, the zinc coating is then metallurgically bonded to the steel.
Galvanizing creates an easy-to-clean surface that can give a maintenance-free life of more than 70 years (depending on the environment it is being used in). Galvanized steel is widely used in applications where corrosion resistance is needed without the cost of stainless steel and is considered superior in terms of cost and life-cycle. It can be identified by the crystallization patterning on the surface (often called a “spangle”).
Galvanized steel can be welded; however, one must exercise caution around the resulting toxic zinc fumes. Hot-dip galvanized steel, is steel covered in a zinc coating to protect the steel from corrosion. Galvanized fumes are released when the galvanized metal reaches a certain temperature.
The galvanizing process involves cleaning the steel and dipping it in a ‘bath’ of molten zinc solution at temperatures around 450 degrees. This temperature varies by the galvanization process used. The liquid zinc adheres to the metal and when you pull the metal out of the bath the zinc hardens. In long-term, continuous exposure, the recommended maximum temperature for hot-dip galvanized steel is 200°C (392°F), according to the American Galvanizers Association.
Galvanized steel is widely used in applications where corrosion protection is needed and can be identified by the crystallized pattern on the surface. The use of galvanized steel at temperatures above this will result in the peeling of the zinc at the intermetallic layer. For many applications, the cost of hot-dip galvanizing is lower than that of applying alternative coatings. It is important to understand not all zinc coatings are created equally; thus, applying any of the information provided about hot-dip galvanizing to other zinc coatings is not accurate or recommended.
Information Source: