Hydrocyanic acid is a highly toxic chemical that is made up of hydrogen cyanide and water. It is also known as prussic acid. HCN is the chemical formula for hydrocyanic acid. Hydrocyanic acid is a colorless liquid with a fast-dissipating vapor that is lighter than air. At temperatures below 26.5°C, it’s a watery white liquid with a faint odor of bitter almonds. The typical commercial material is 96–99 percent pure and water soluble. The solution is mildly acidic and light sensitive. Hydrogen cyanide polymerizes when it isn’t completely pure or stable.
Commercially, hydrocyanic acid is commonly sold as an aqueous solution containing 2 to 10% hydrogen cyanide. It has a strong odor that reminds me of bitter almonds. Amines, oxidizers, acids, sodium hydroxide, calcium hydroxide, sodium carbonate, caustic compounds, and ammonia are all reactants for HCN. Hydrogen cyanide aqueous solutions decompose slowly to form ammonium format. It’s a highly toxic colorless gas with a distinct bitter almond scent. Because it is a hazardous transparent liquid, it is not permitted to be stored or transported.
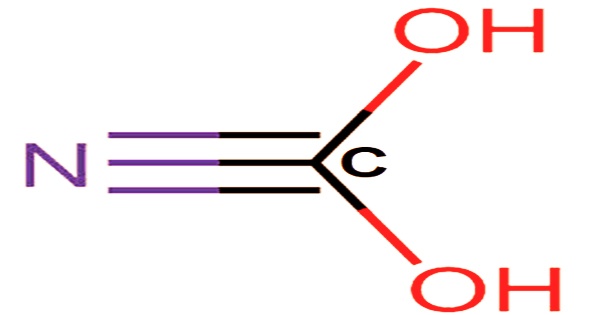
In 1704, HCN was isolated from Prussian blue, a blue dye. HCN can be found in pitted fruits like cherries, apricots, and bitter almonds, which are used to make almond oil and flavoring. Its molecular formula is written as CHN and its molar mass is 27.03 g/mol. The carbon and nitrogen atoms in hydrogen cyanide form a triple bond, making it a simple planar molecule. Fumigation, electroplating, mining, and the production of synthetic fibers, plastics, dyes, and pesticides all use HCN. It is also used as an intermediate in chemical syntheses.
HCN is found in the pits of some fruits, including cherries, apples, and apricots. Small amounts of cyanohydrins, which form HCN, are found in the fruit pits. Workers in the electroplating, metallurgical, firefighting, steel manufacturing, and metal cleaning industries are exposed to cyanide. Humans are also exposed to cyanide from industrial organic chemical wastewater discharges, iron and steel works, and wastewater treatment facilities. On a laboratory scale, HCN is made by mixing acids with alkali metal cyanide salts (such as NaCN, KCN, etc.):
HCl + NaCN → HCN + NaCl
When hydrocyanic acid reacts with a base such as sodium hydroxide, sodium cyanide and water are formed. The chemical equation is given below.
HCN + NaOH → NaCN + H2O
At room temperature, HCN readily evaporates (or boils), and the vapors are slightly lighter than air. It’s water soluble, so it reacts with amines, oxidizers, acids, sodium hydroxide, calcium hydroxide, sodium carbonate, caustics, and ammonia. Methane and ammonia are oxidized at around 1200 °C, over a platinum catalyst in the main industrial preparation:
2 CH4 + 2 NH3 + 3 O2 → 2 HCN + 6 H2O
Hydrogen cyanide is produced through the controlled oxidation of ammonia–methane mixtures and the catalytic decomposition of formamide. It is a combustion by-product of nitrogen-containing materials such as wool, silk, and plastics, and it can be produced by treating cyanide salts with acid. Hydrocyanic acid has a density of 0.687 g/mL, and boils slightly above room temperature, at 25.6 °C (78.1 °F). It has a distinct bitter almond odor that is used to detect the presence of this highly toxic substance.
Enzymatic hydrolysis of nitriles and related compounds also produces HCN. Coke oven and blast furnace operations produce hydrogen cyanide gas as a byproduct. Hydrogen cyanide has a wide range of industrial applications. It forms potassium cyanide and water when it reacts with potassium hydroxide. The chemical equation is given below.
HCN + KOH → H2O + KCN
HCN is used in large quantities to make a variety of chemical products and intermediates for organic synthesis. HCN is often used as a disinfectant in the form of a gas, or cellulosic disks impregnated with HCN. Cyanides are commonly used in ore processing and metal treatment. It’s also used as a figment to destroy pests like rodents in warehouses, grain storage bins, greenhouses, and ship holds because of its high toxicity and ability to penetrate tight spaces.
Amines, oxidizers, acids, sodium hydroxide, calcium hydroxide, sodium carbonate, caustic compounds, and ammonia are all reactants for HCN. Anunonium formate is formed when hydrogen cyanide aqueous solutions decompose slowly. In some cases, it is preferable to produce hydrogen cyanide only as required, avoiding handling and storage issues. HCN is a chemical that is used to produce cyanide salts, acrylonitrile, and dyes. It is also used as a horticultural fumigant.
A few hundred parts per million of hydrogen cyanide in the air can kill a human in less than an hour, and if ingested, it can kill almost instantly. When high concentrations of HCN gas (around 5.6 percent) are exposed to air, it becomes explosive. Asphyxia occurs when oxygen is not available to the tissues, resulting in death. Exposure to HCN is more dangerous for them. Carbon monoxide-induced incapacitation in rats was accelerated by HCN and nitric oxide. Carbonyl hemoglobin concentrations of 42.2–49 percent caused incapacitation, while CO alone caused the same effect at 50–55 percent carbonyl hemoglobin.
Information Sources: