Hydrogen peroxide (H2O2) is a potent oxidizing agent that is commonly used in industry and medicine. It’s a very pale blue liquid that’s much more viscous than water in its purest form. It’s a bleaching agent, an oxidizer, and an antiseptic. It’s usually sold as aqueous solutions with concentrations of 3, 30, or 90% by weight. The 3% solution is used as a topical antiseptic and cleaning agent, as well as a component in mouthwashes, dentifrices, and sanitary lotions; the 30% solution is used as an efficient bleaching agent and other industrial applications; and the 90% solution is used as a vigorous oxidizer of rocket fuels. Hydrogen peroxide exerts its oxidizing activity and produces free radicals when rinsed and gargled or applied topically, causing oxidative damage to proteins and membrane lipids. This has the ability to inactivate and kill pathogens, as well as prevent infection from spreading.
Concentrated hydrogen peroxide, also known as “high-test peroxide,” is a reactive oxygen species that has previously been used as a rocket propellant. The O-O bond dominates its chemistry. Hydrogen peroxide solutions are unstable in the absence of stabilizing agents (e.g., phosphates, tin), and decompose upon standing, agitation, exposure to light, or heating. Many oxidizing and reducing agents react violently with hydrogen peroxide. To the skin, concentrated liquids are extremely caustic. Hydrogen peroxide is also used as a bleaching agent in some food products and can be used in over-the-counter (OTC) first aid antiseptics.
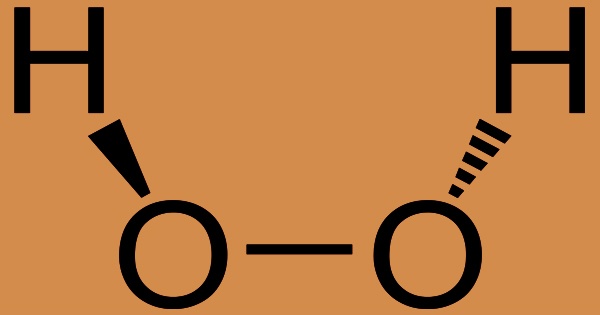
At room temperature, hydrogen peroxide is a colorless liquid with a bitter odor. In the air, small quantities of gaseous hydrogen peroxide exist naturally. Hydrogen peroxide has proven to be a valuable antimicrobial agent in addition to its efficacy as a bleach. This latter property has been used as a milk and whey preservative in some countries. It’s normally held in a dark colored container with a stabilizer in a weakly acidic solution. The human body produces hydrogen peroxide, which is present in biological processes. Peroxidases are enzymes that use or decompose hydrogen peroxide.
With the release of heat, hydrogen peroxide decomposes quickly to oxygen and water. It is a strong oxidizing agent that can induce spontaneous combustion when it comes into contact with organic material, despite being nonflammable. H2O2 has been determined to have a boiling point of 150.2 °C (302.4 °F), which is around 50 °C (90 °F) higher than water. In fact, if hydrogen peroxide is heated to this temperature, it will undergo potentially explosive thermal decomposition. It can be distilled safely at lower temperatures and under lower pressure.
Thenard was the first to make hydrogen peroxide in 1818, and it has a wide range of industrial applications. Many households have low concentrations (3-9 percent) for medical purposes and as a clothes and hair bleach. Higher concentrations of hydrogen peroxide are used in industry as a bleach for textiles and paper, as a part of rocket fuels, and for the manufacture of foam rubber and organic chemicals. Aqueous solutions are used for bleaching fabrics, silks, furs, feathers, and hair; as a dough conditioner; as a bleaching and oxidizing agent in foods; for washing metals; as a laboratory reagent for oxidation; as an antiseptic; in sewage and wastewater treatment; and in the preparation of inorganic and organic peroxides. An 80% concentrated solution is used in rocket propulsion.
At low temperatures, stabilized hydrogen peroxide appears as a crystalline solid with a strongly pungent, unpleasant odor. Hydrogen peroxide is a colorless liquid with a bitter taste. It melts at 150.2°C and freezes at –0.43°C. It has a vapor pressure of 9.9 torr at 50°C and 121.5 torr at 100°C. It has a viscosity of 1.245 centipoise at 20°C and a surface tension of 80.4 dyn/cm at 20°C. It is miscible with water. It is soluble in ether; at 25°C, the densities of 30 percent, 70 percent, and 90 percent H2O2 solutions are 1.1081, 1.2839, and 1.3867 g/mL, respectively; their freezing points are –25.7°C, –40.3°C, and –11.5°C, respectively; and their boiling points are 106.2°C, 125.5°C, and 141.3°C, respectively; and their pKa at 25°C
The anthraquinone process, which was developed and patented by the German chemical company BASF in 1939, is now almost exclusively used to produce hydrogen peroxide. Electrolysis was the dominant method of processing from 1920 to 1950. The peroxydisulfate ion (S2O8 2-) was formed by passing an electric current through sulfuric acid, which was then hydrolyzed to H2O2: 2H2O + S2O82- (aq) 2H2SO4–(aq) + H2O2 (aq). The relatively high cost of energy in this system prompted a quest for a more cost-effective manufacturing method.
To convert anthraquinone to hydroquinone, a metal catalyst such as palladium or nickel is used, followed by autooxidation in air to create hydrogen peroxide. The anthraquinone and hydrogen peroxide are separated; the former is recycled for use in the process again, while the latter is purified. Several methods can be used to create small quantities of hydrogen peroxide that are detectable. Electrolysis of dilute acid around the cathode creates small quantities of hydrogen as oxygen is bubbled around it. It can also be created by exposing water to UV rays from a mercury lamp or an electric arc while it is stored in a UV transparent tank (e.g. quartz).
Heat 2-propanol with oxygen at 100°C under 10 to 20 atm pressure to make hydrogen peroxide: (CH3)2CHOH (CH3)2C(OH)OOH → CH3COCH3 + H2O2; H2O2 is also provided by partial oxidation of hydrocarbons in the vapor phase. However, many by-products are made, and their separation makes the process difficult and costly. Textiles, wood pulp, hair, fur, and other materials are bleached and deodorized with hydrogen peroxide; as a source of organic and inorganic peroxides; pulp and paper industry; plasticizers; rocket fuel; foam rubber; manufacture of glycerol; antichlor; dyeing; electroplating; antiseptic; laboratory reagent; epoxidation; hydroxylation; oxidation and reduction; viscosity control for starch and cellulose derivatives; refining and cleaning metals; bleaching and oxidizing agent in foods; neutralizing agent in wine distillation; seed disinfectant; substitute for chlorine in water and sewage treatment.
In 1994, global H2O2 production was about 1.9 million tonnes, rising to 2.2 million tonnes in 2006, with the majority of it produced at a concentration of 70% or less. Bulk 30 percent H2O2 sold for about 0.54 USD/kg in that year, which was equivalent to US$1.50/kg (US$0.68/lb) on a “100 percent basis.” Peroxy compounds or peroxyhydrates are formed when hydrogen peroxide reacts with borates, carbonates, pyrophosphates, sulfates, silicates, and a variety of organic carboxylic acids, esters, and anhydrides. Several of these compounds are solids that readily hydrolyze to create hydrogen peroxide in solution.
Information Sources: