Researchers report the first direct observation of a key intermediate product created during hydrocarbon breakdown, which is crucial for understanding climate change and combustion.
Combustion is a high-temperature exothermic (heat-releasing) redox (oxygen-adding) chemical process that creates oxidized, frequently gaseous products in a mixture known as smoke. It’s a synonym for “burning.” Fuel is heated and interacts with oxygen in a combustion process.
Chemists have known for a long time that a certain class of molecules essential to understanding both climate change and combustion chemistry must exist. But, until recently, they hadn’t been able to track it down and investigate it.
Researchers from the Department of Energy’s (DOE) Argonne National Laboratory and the University of Pennsylvania reveal for the first time that they have directly observed and investigated a prototype version of this class of molecules in a publication published in the journal Science.
Carbon-centered hydroperoxyalkyl radical is the chemical name for it. Chemists commonly refer to it as QOOH. It’s a byproduct of reactions that’s vital for climate change models and the development of more fuel-efficient combustion engines.
The findings of this study might aid in the development of more efficient and low-polluting engines, as well as a better knowledge of the oxidation processes that polluting pollutants experience in the environment. They can even be used to better understand the atmospheric responses of naturally occurring volatile organic chemicals across the planet.
Although energy is absorbed during the reaction, it is released when stronger bonds are created, resulting in the formation of carbon dioxide (CO2) and water (H2O). While the fuel contributes to the reaction’s energy, it is little in contrast since the chemical bonds in the fuel are equivalent to the energy of the bonds in the products.
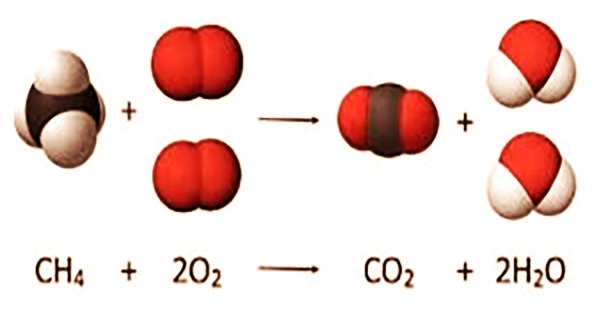
While QOOH has been postulated for a long time, it is difficult to see firsthand due to its rapid degradation.
“This intermediate product is a switchyard controlling various subsequent steps that are really important for the propagation of this chemistry,” said Marsha I. Lester, Christopher H. Browne Distinguished Professor of Chemistry at the University of Pennsylvania. “But prototypical QOOH intermediates have not been directly observed, so there were critical pieces missing about how this network of chemical reactions occurs.”
The QOOH molecule is produced as a byproduct of volatile organic compound processes. They’re commonly emitted by trees and industrial processes, and they’re important components of gasoline. These chemicals are also released into the environment as a result of the usage of home items and even construction materials.
There is always a competition between the QOOH splitting into smaller molecules or oxygen reacting with the QOOH. Understanding that competition is essential to much of what happens in atmospheric and combustion chemistry.
Stephen Klippenstein
In both combustion and the environment, a comparable route for the interaction of volatile organic molecules with oxygen at low temperatures exists. Whether or whether this oxidation process occurs is determined by the QOOH molecule.
“There is always a competition between the QOOH splitting into smaller molecules or oxygen reacting with the QOOH,” explained Stephen Klippenstein, Argonne Distinguished Fellow in the Chemical Sciences and Engineering division. “Understanding that competition is essential to much of what happens in atmospheric and combustion chemistry.”
This mysterious prototype molecule was initially isolated and then explored by the researchers. Earlier experiments had only seen the oxidation reaction’s ultimate products, but the present study was the first to see this key intermediate product.
The researchers also looked at how the lifespan and decay rate of QOOH varies with its energy. “We have been making predictions of these quantities for years, but had no idea how good they were,” said Klippenstein. “We found out they had some flaws we could fix.”
As a consequence, team members revised their theoretical model, and the forecast and experimental findings were now extremely accurate. They’ll put this newfound understanding to good use in future research of related compounds involved in environmental and combustion chemistry.
The study was published in the journal Science with the title “Watching a hydroperoxyalkyl radical (•QOOH) dissolve.” In addition to Klippenstein and Lester, authors include Anne S. Hansen, Trisha Bhagde, Kevin B. Moore III, Daniel R. Moberg, Ahren W. Jasper, Yuri Georgievskii and Michael F. Vansco.
The DOE’s Office of Basic Energy Sciences, the National Science Foundation, and the US Army Research Office provided funding. The computational capabilities of Bebop, Argonne’s Laboratory Computing Resource Center’s high-performance computing cluster, were used for the theoretical simulations.