A hypohalous acid is an oxyacid consisting of a hydroxyl group single-bonded to any halogen. It is an oxyacid of a halogen (fluorine, chlorine, bromine, iodine, or astatine) possessing the general chemical formula HOX, where X is the halogen atom. Examples include hypofluorous acid, hypochlorous acid, hypobromous acid, and hypoiodous acid. The conjugate base is a hypohalite. It is an acid HXO derived from halogens and including hypochlorous acid, hypobromous acid, and hypoiodous acid. They can be formed by reacting the corresponding diatomic halogen molecule (F2, Cl2, Br2, I2) with water in the reaction:
X2 + H2O ⇌ HXO + HX
This also results in the corresponding hydrogen halide, which is also acidic.
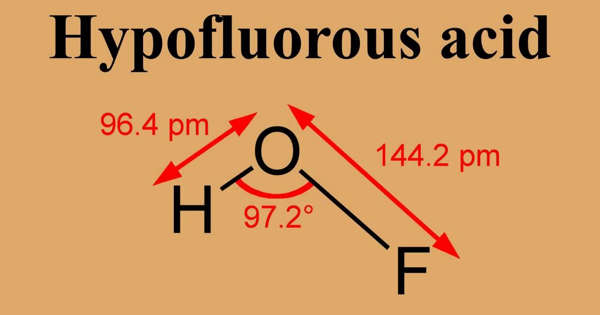
The compound has been characterized in the solid phase by X-ray crystallography as a bent molecule with an angle of 101°. The O–F and O–H bond lengths are 144.2 and 96.4 picometres, respectively. The solid framework consists of chains with O–H···O linkages. The structure has also been analyzed in the gas phase, a state in which the H–O–F bond angle is slightly narrower (97.2°).
Stability
Hypohalous acids tend to be unstable. The chemical behavior of hypofluorous acid (HOF) is dramatically different from the heavier hypohalous acids which, as a group, exhibit similar properties. Only hypofluorous acid has been isolated as a solid, and even it is explosive at room temperature. These differences are attributed primarily to the high electronegativity and small size of the fluorine atom, which cause HOF to be an extremely strong oxidant with an anomalously weak O-F bond.
Hypochlorous acid cannot be prepared in anhydrous form. It is any oxyacid of a halogen of the general formula HOX. Hypobromous acid, hypoiodous acid, and their conjugate bases (hypobromite and hypoiodite) are also unstable, undergoing disproportionation reactions like
3BrO–(aq) → 2Br–(aq) + BrO–3(aq)
and
3HIO → 2HI + HIO3
that results in the corresponding hydrogen halides/halide ions and halic acids/halates. HOF, therefore, acts primarily as an oxygenating and hydroxylating agent, whereas the other hypohalous acids are electrophilic halogenating agents.
HOF is only marginally stable at room temperature, decomposing to HF and O2 with a half-life of about 30 min at room temperature. HOF also reacts rapidly with water, the predominant products being hydrogen peroxide and HF under acidic conditions and O2 and HF under alkaline conditions.
Uses
Hypochlorous acid and hypobromous acid are each dissolved in water in order to sanitize it, hypochlorous acid in swimming pools, and hypobromous acid in hot tubs and spas.
- It is used to convert alkenes to chlorohydrins.
- It is used in cosmetics such as baby products.
- It is used in swimming pools.
- It is used to generate sufficient quantities of safe disinfectant.
- It is used in marine sanitation devices to convert seawater into HOCl.
Information Source: