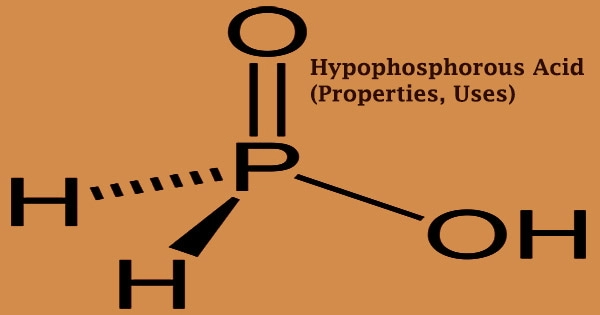
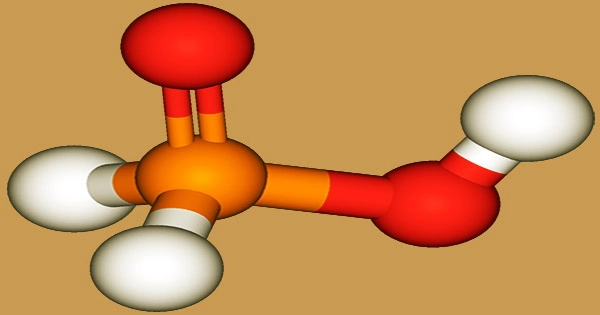
Hypophosphorous acid (HPA), also known as “hypophosphite”, is a colorless oil or deliquescence crystal that is an essential fine chemical product. With the chemical formula H3PO2, it is a phosphorus oxyacid and a potent reducing agent. Phosphinic acid is a phosphorus oxoacid composed of a single pentavalent phosphorus covalently connected to two hydrogens and a hydroxy group through single bonds, as well as a double bond to an oxygen. It’s a phosphorus oxoacid that belongs to the phosphinic acids family.
The conventional formula for this acid is H3PO2, but a more descriptive abbreviation is HOP(O)H2, which emphasizes its monoprotic nature. Phosphoric acid is primarily used as a reducing agent in electroless plating to minimize resin discolouration. It may also be utilized as an esterification reaction catalyst and a refrigerant, particularly for the manufacturing of sodium hypophosphite, a high purity product.
The main tautomer HOP(O)H2 is in equilibrium with the minor tautomer HP(OH)2; the minor tautomer is sometimes referred to as hypophosphorous acid, while the major tautomer is referred to as phosphinic acid. There are numerous ways for preparation; the most prevalent industrial procedures are ion exchange resin and electrodialysis. Because of the quick oxidation of the hypophosphorous acid to phosphoric acids (and elemental phosphorus) and its disproportionation to phosphine and phosphorous acid, the pure acid cannot be extracted simply by evaporating the water.
Pierre Louis Dulong (1785–1838), a French scientist, created hypophosphorous acid for the first time in 1816. A two-step technique is used to make the acid in the industrial setting: To begin, elemental phosphorus interacts with alkali and alkaline earth hydroxides to form hypophosphites in an aqueous solution:
P4 + 4 OH− + 4 H2O → 4 H2PO−2 + 2 H2
Diethyl ether, (C2H5)2O, can be used to extract the pure acid from its aqueous solution. At 26.5 °C (79.7 °F), pure hypophosphorous acid generates white crystals that melt. To obtain free hypophosphorous acid, the purified material is treated with a strong, non-oxidizing acid (typically sulfuric acid):
H2PO−2 + H+ → H3PO2
HPA is a colorless oil or deliquescent crystal with a melting point of 26.5°C. The specific gravity (relative density) is 1.439 (solid, 19°C). HPA is typically delivered in a 50% aqueous solution. Because the acid readily oxidizes to phosphorous acid and phosphoric acid, as well as disproportionates to phosphorous acid and phosphine, anhydrous acid cannot be created by simple evaporation of water.
Hypophosphorous acid is a monoprotic oxyacid because it contains just one hydrogen atom linked to oxygen in its electronic structure. It’s a weak acid that only produces one type of salt: hypophosphites. It is soluble in water, ethanol, and ether, and it may be combined with water, ethanol, or acetone in any proportion. It quickly deliquesces into a syrupy liquid in the air, and the aqueous solution is acidic.
Continuous extraction of aqueous solutions with diethyl ether can provide pure anhydrous hypophosphorous acid. Hypophosphorous acid is a strong acid with a Ka = 10-2 (25°C) and is rather stable at ambient temperature; however, above 130°C, the disproportionation reaction can occur, resulting in the formation of phosphine and phosphorous acid:
2H3PO2 = H3PO4 + PH3
Because hypophosphites have a tendency to convert metal cations back to bulk metal, most metal-hypophosphite complexes are unstable. HPA can break down into very poisonous phosphine gas or possibly explode when heated to a high temperature. It’s a caustic substance. Because hypophosphorous acid is not absorbed, it is frequently added to soft drinks. So the danger is low, but hypophosphite, especially significant hypophosphite, might cause gastrointestinal problems.
It is occasionally employed as an additive in Fischer esterification processes, where it avoids the development of colored impurities, due to its capacity to operate as a moderate reducing agent and oxygen scavenger. Hypophosphorous acid is used as a reducing agent in electroless plating and to avoid phosphoric acid resin discoloration. It’s a refrigerant and an esterification catalyst.
Hypophosphite is made from hypophosphorous acid, and sodium salts, manganese salts, and iron salts are commonly utilized as nourishing ingredients. It’s also utilized in medicine and as a reducing agent for arsenic, tellurium, and tantalum, niobium, and other reagent separation. It may decrease a variety of transition metal ions (e.g., Co, Cu, Ag, Mn, and Pt), although it is most typically employed to reduce nickel. It may be used to make sodium hypophosphite, calcium phosphate, and other hypophosphites since it is a powerful reducing agent.
Information Sources: