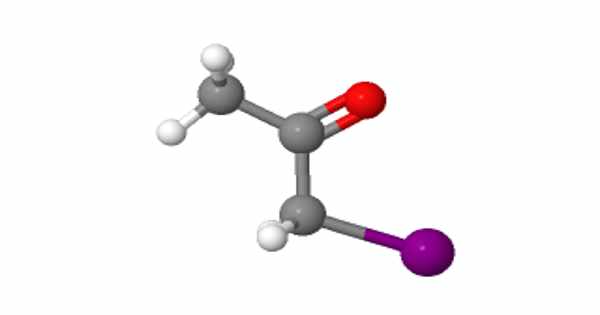
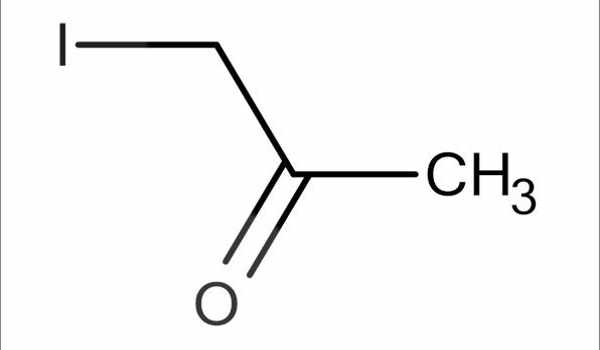
Iodoacetone has the chemical formula C3H5IO and is an organoiodine compound. The molecule of iodoacetone has a total of ten atoms (s). There is 5 Hydrogen atom(s), 3 Carbon atom(s), 1 Oxygen atom(s), and 1 Iodine atom(s) in this compound (s). Under normal conditions, the substance is a colorless liquid that is soluble in ethanol.
The haloform reaction can halogenate acetone in the presence of a halide and an acid or base, though it can theoretically stop before exhaustive halogenation of an acetone terminal carbon.
Properties
- Group of substances: organic
- Physical appearance: yellow liquid
- Molar/atomic mass: 183.976
- Solubility (g/100 g of solvent): ethanol: soluble
- Vapour pressure (Torr): 12 (62°C)
Chemical Formula
The Iodoacetone chemical formula shown is based on the molecular formula, which indicates the numbers of each type of atom in a molecule without structural information, as opposed to the empirical formula, which provides the numerical proportions of atoms of each type.
The above chemical formula serves as the foundation for stoichiometry in chemical equations, which is the calculation of the relative quantities of reactants and products in chemical reactions. The law of conservation of mass states that in a chemical reaction, the quantity of each element given in the chemical formula does not change. As a result, based on the chemical formula, each side of the chemical equation must represent the same quantity of any particular element.
In the chemical structure of Iodoacetone, the carbon atoms are implied to be located at the corner(s), and hydrogen atoms attached to carbon atoms are not indicated – each carbon atom is assumed to be associated with enough hydrogen atoms to provide the carbon atom with four bonds.
Synthesis
The reaction of acetone and iodine produces iodoacetone. The reaction is typically acid-catalyzed and first order with respect to acetone and the acid catalyst:
C3H6O + I2 → HI + C3H5IO
Emmerling’s method was used to make iodoacetone from commercial chloroacetone and potassium iodide. At 35° and 2 mm, the product was washed, dried, and distilled. Decomposed iodine was removed by treating the liquid with copper overnight in a cold room, and the remaining liquid was filtered and dissolved in a solvent. When such a solution was hydrolyzed with aqueous sodium hydroxide, the resulting iodide titration yielded approximately 9S percent of the value calculated from the weight of iodoacetone dissolved. Solutions could be stored at 6 for several weeks before being discarded because they began to develop the color of free iodine.