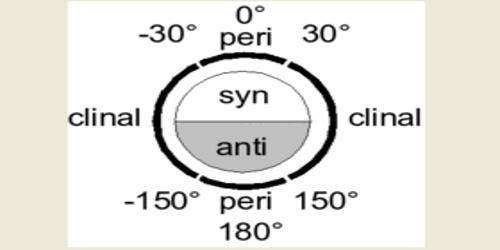
In stereochemistry, the Klyne–Prelog system for describing conformations about a single bond offers a more systematic means to unambiguously name complex structures, where the torsional or dihedral angles are not found to occur in 60° increments. Torsion angles play an important in discussions of stereochemical problems and so the terminology relating to them must be precise as far as possible. Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The torsion angle is no longer a multiple of 60° but can have intermediate values. In fact, even for the tetrahedral compounds, in most cases, the torsion angles are different from the ideal used values. Klyne notation views the placement of the substituent on the front atom as being in regions of space called anti/syn and clinal/periplanar relative to a reference group on the rear atom. A plus (+) or minus (–) sign is placed at the front to indicate the sign of the dihedral angle. Anti or syn indicates the substituents are on opposite sides of the same side, respectively. A general and detailed method of nomenclature has been worked out by Klyn and Prelog (1960) to describe steric relationships across a single bond in a molecule or part of the molecule.
Clinal substituents are found within 30° of either side of a dihedral angle of 60° (from 30° to 90°), 120° (90°–150°), 240° (210°–270°), or 300° (270°–330°). Periplanar substituents are found within 30° of either 0° (330°–30°) or 180° (150°–210°). Juxtaposing the designations produces the following terms for the conformers of butane: gauche butane is syn-clinal (+sc or –sc, depending on the enantiomer), anti butane is anti-periplanar, and eclipsed butane is syn-periplanar.
In addition to the Cahn-Ingold system, there are two ways of experimentally determining the absolute configuration of an enantiomer:
- X-ray diffraction analysis. Note that there is no correlation between the sign of rotation and the structure of a particular enantiomer.
- Chemical correlation with a molecule whose structure has already been determined via X-ray diffraction.