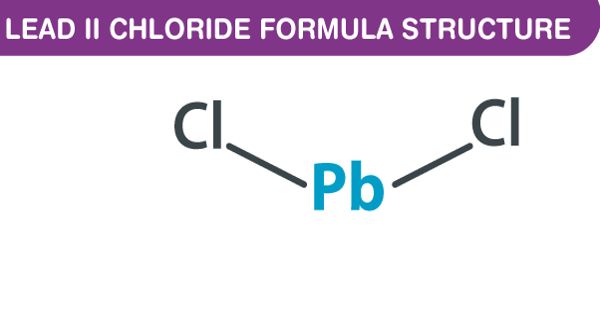
Lead(II) chloride (PbCl2) is an inorganic compound that is a white solid under ambient conditions. It is an inorganic chloride consisting of two chlorine atoms covalently bound to a central lead atom. It is poorly soluble in water. Lead(II) chloride is one of the most important lead-based reagents. It is a lead coordination entity and an inorganic chloride. It also occurs naturally in the form of the mineral cotunnite.
Properties
Lead II chloride has the appearance of a white solid. It is a colorless crystalline solid. It does not dissolve well in water. It appears in the form of white crystalline powder or white orthorhombic needles. It can dissolve in solutions that have chloride ions in them. The boiling point of this inorganic compound is at a temperature of 950°C. Furthermore, the melting point of lead II chloride is 501°C. It reacts with molten sodium nitrite to make lead(II) oxide.
- Compound Formula: Cl2Pb
- Molecular Weight: 278.1
- Appearance: white odorless solid
- Melting Point: 501° C (933.8° F)
- Boiling Point: 950° C (1,742° F)
- Density: 5.85 g/cm3
- Solubility in H2O: N/A
- Exact Mass: 277.914 g/mol
Preparation
Lead(II) chloride is made by reacting sodium chloride or hydrochloric acid with lead nitrate. It can also be made by reacting lead(IV) oxide with hydrochloric acid. It can also be made by reacting chlorine with lead.

Occurrence
PbCl2 occurs naturally in the form of the mineral cotunnite. It is colorless, white, yellow, or green with a density of 5.3–5.8 g/cm3. The hardness on the Mohs scale is 1.5–2. The crystal structure is orthorhombic dipyramidal and the point group is 2/m 2/m 2/m. Each Pb has a coordination number of 9. Cotunnite occurs near volcanoes: Vesuvius, Italy; Tarapacá, Chile; and Tolbachik, Russia.
Uses
Experts use lead II chloride in the production of infrared transmitting glass. Furthermore, experts also use this compound in aurene glass, and ornamental glass.
Molten PbCl2 is used in the synthesis of lead titanate and barium lead titanate ceramics by cation replacement reactions:
x PbCl2(l) + BaTiO3(s) → Ba1−xPbxTiO3 + x BaCl2
PbCl2 is used in the production of infrared transmitting glass, and ornamental glass called aurene glass. Aurene glass has an iridescent surface formed by spraying with PbCl2 and reheating under controlled conditions.
Pb is used in HCl service even though the PbCl2 formed is slightly soluble in HCl.
A basic chloride of lead, PbCl2·Pb(OH)2, is known as Pattinson’s white lead and is used as pigment in white paint.
PbCl2 is an intermediate in refining bismuth (Bi) ore. The ore containing Bi, Pb, and Zn is first treated with molten caustic soda to remove traces of arsenic and tellurium.
Toxicity
Like other soluble lead compounds, exposure to PbCl2 may cause lead poisoning.
Information Source: