Lead(II) oxide, also known as lead monoxide, is a yellow tetragonal powder, soluble in acetone, nitric acid, caustic soda, and ammonium chloride, insoluble in water and ethanol. It is an inorganic material with the PbO molecular formula. In two polymorphs, red, with a tetragonal crystal structure, and yellow, with an orthorhombic crystal structure, lead(II) oxide occurs. As rare minerals, both forms occur naturally. Modern PbO uses, including computer parts, are mainly in lead-based industrial glass and industrial ceramics. It is an oxide of amphoteric acid. The red form is known as “Litharge” and “Massico” is known as the yellow form.
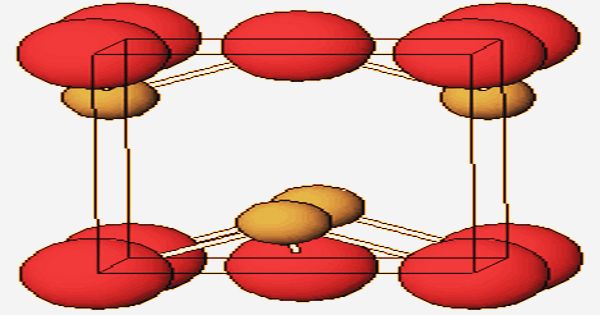
Crystal structure of Lead(II) Oxide
Lead(II) oxide (PbO) can be prepared at approximately 600 °C (1,100 °F) by heating lead metal in the air. It is also the end result of the oxidation of other lead oxides in the air at this temperature:
PbO2 293 °C → Pb12O19 351 °C → Pb12O17 375 °C → Pb3O4 605 °C → PbO
To obtain the finished lead oxide, the oxide components are molded. As an intermediate product in the processing of raw lead minerals into a metallic lead, PbO is developed on a large scale. Galena (lead(II) sulfide) is the typical lead ore. The sulfide is transferred to the oxide at a temperature of about 1,000 °C (1,800 °F):
2 PbS + 3 O2 → 2 PbO + 2 SO2
Metallic lead is obtained at about 1,200 °C (2,200 °F) by lowering PbO with carbon monoxide:
PbO + CO → Pb + CO2
Two crystalline modifications, the reddish or orange-red alpha form, known as litharge, and the yellow beta form, massicot, are displayed by the oxide. As determined by X-ray crystallography, a pyramidal four-coordinate lead core is found in both polymorphs, tetragonal and orthorhombic. Tetragonal crystals constitute the alpha form, while the beta alteration is a yellow amorphous orthorhombic crystal structure powder.
The alpha form is stable at normal temperatures, converting when heated at 489 °C to the beta form; density 9.35 g / cm3 (beta form); Moh’s hardness 2 (alpha form); oxide melts at 888 °C; vaporizes at 1,472 ° C with decomposition; vapor pressure 1 torr at 943 °C and 5 torr at 1,039 °C; almost insoluble in water (alpha form solubility is 17 mg / L at 20 °C and beta type 23 mg / L at 22 °C)

Lead(II) Oxide
The PbO can be modified by managed heating and cooling from massicot to litharge, or vice versa. The tetragonal shape is usually red or orange, while the orthorhombic shape is usually yellow or orange, but the form is not a very good indicator of color. Lead monoxide has poor forces of oxidization or reduction. Redox reactions will still occur, however. The majority of compounds are partially soluble or insoluble in water in this class. When soluble in water, the solutions are typically neither highly acidic nor highly basic.
Since it remains the key component of automotive lead-acid batteries, the use of lead, and thus the manufacturing of PbO, correlates with the amount of vehicles. It is used primarily in electron tubes, image tubes, optical glass, lead glass anti-X-ray, and rubber goods that are radiation-resistant. Used in metal lead smelting and preparation of lead glass, lead compound, catalyst, and drier for paint. Lead(II) oxide is also used as decomposing silicate and metallurgy fluxing agents and paint driers and is also used for gold and silver determination and amino acid precipitation. In the glass and rubber industry, it can be mixed with glycerol to create strong materials such as adhesives.
A mixture of PbO with glycerine sets to stiff, waterproof cement that was used to join aquarium flat glass sides and bottoms, and was once often used in window frames to seal glass panels. In organic synthesis, it is used in many condensation responses. In a video camera tube, called the Plumbicon, PbO is the input photoconductor. A modified Ball Mill process in which high purity lead balls put in the mill are partially oxidized to create black or grey oxide is also provided by lead monoxide. Alkaline dehydration of lead hydroxide, Pb(OH)2, will prepare both the red and yellow form of the oxide.
Information Sources: