The carbonate salt of lithium, a soft alkali metal with antimanic and hematopoietic properties, is lithium carbonate. It is an inorganic compound with the formula Li2CO3 that is the lithium salt of carbonate. It has the appearance of a white powder. This white salt is widely used in the processing of metal oxides and as a medication to treat mood disorders. It is an inorganic salt that is used in the chemical industry to oxidize metals and in the pharmaceutical industry to treat bipolar disorder.
It is on the World Health Organization’s List of Essential Medicines for the treatment of bipolar disorder, which includes the most important medications required in a basic health system. It is included on the World Health Organization’s (WHO) model list of the Most Essential Medicines required in a basic health care system.
Structure
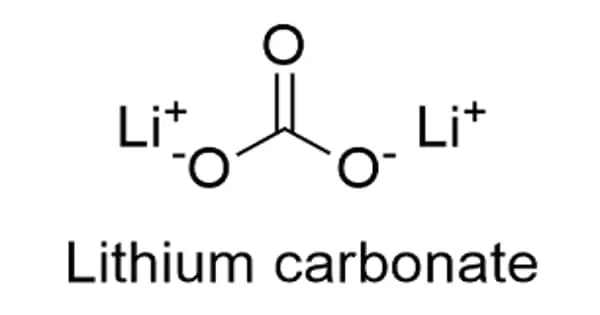
The chemical formula for lithium carbonate is Li2CO3, and its molar mass is 73.89 g mol-1. The molecule is formed by the lithium cation Li+ carbonate anion CO3-2 and has a monoclinic crystal structure. In the common representations for organic molecules, their chemical structure can be written as follows.
In both low-fire and high-fire ceramic glazes, lithium carbonate is a common ingredient. It combines with silica and other materials to form low-melting fluxes. Its alkaline properties make it suitable for modifying the state of metal oxide colorants in glaze, particularly red iron oxide (Fe2O3).

Physical properties
Lithium carbonate is a white powder with no odor. It has a density of 2.11 g mL-1. The melting point of lithium carbonate is 724 degrees Celsius, and its boiling point is 1310 degrees Celsius. In hot water, acetone, ammonia, and ethanol, it is insoluble. It is insoluble in cold water (its solubility in water decrease with the increasing temperature). Acetic acid dissolves it.
- Molecular Weight: 73.9
- Appearance: White powder
- Melting Point: 618-723 °C
- Boiling Point: 1310 °C (dec.)
- Density: 2.11 g/cm3
- Solubility in H2O: 1.29 g/100 mL (25 °C)
- Specific Heat: 97.4 J/mol·K
Preparation
Although lithium carbonate can be synthesized chemically, it is most commonly extracted from minerals. There are several chemical methods for producing lithium carbonate, but the most common is a reaction in water between lithium hydroxide or lithium chloride and sodium carbonate, which results in a precipitate of lithium carbonate:
2 LiOH + Na2CO3 → Li2CO3 + 2 NaOH
2 LiCl + Na2CO3 → Li2CO3 + 2 NaCl
Unlike sodium carbonate, which has at least three hydrates, lithium carbonate only exists as an anhydrous compound. In comparison to other lithium salts, it has low water solubility. The extraction of lithium from aqueous extracts of lithium ores takes advantage of this low solubility. Its apparent solubility increases 10-fold under mild carbon dioxide pressure; this effect is due to the formation of the more soluble metastable bicarbonate:
Li2CO3 + CO2 + H2O ⇌ 2 LiHCO3
The extraction of lithium carbonate at high pressures of CO2 and its precipitation upon depressurizing is the basis of the Quebec process. It can also be purified by exploiting its diminished solubility in hot water. Thus, heating a saturated aqueous solution causes crystallization of Li2CO3.
Lithium carbonate, and other carbonates of group 1, do not decarboxylate readily. Li2CO3 decomposes at temperatures around 1300 °C.
Occurrence
Lithium carbonate can be found in ores along with other minerals. Because it is insoluble in water, it is easily extracted, and hot water is used to separate it from other chemical compounds found in ores.
Uses
Lithium carbonate is a critical industrial chemical. Its primary application is as a precursor to compounds used in lithium-ion batteries. Glasses made from lithium carbonate can be used in ovens.
- Rechargeable batteries
Lithium carbonate (and lithium hydroxide) is primarily used as a precursor to lithium compounds used in lithium-ion batteries. In practice, lithium compounds are used to make two components of the battery: the cathode and the electrolyte.
- Medical uses
In 1843, lithium carbonate was introduced as a new solvent for bladder stones. Some doctors recommended lithium salt therapy in 1859 for a variety of ailments, including gout, urinary calculi, rheumatism, mania, depression, and headache. John Cade discovered the anti-manic effects of lithium ions in 1948. It is a psychiatric medication used to treat mania, which is the elevated phase of bipolar disorder.
Health effects/safety hazards
Ingestion of large amounts of lithium carbonate can be extremely toxic. It can also cause side effects and diseases like nephrogenic diabetes insipidus. It is not combustible.