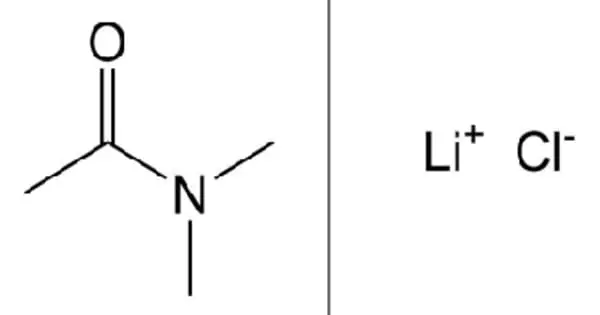
Lithium chloride, abbreviated LiCl, is a chemical compound. It is a metal chloride salt with a counterion of Li(+). The salt is a typical ionic compound (with some covalent properties), but the small size of the Li+ ion gives rise to properties not seen in other alkali metal chlorides, such as exceptional solubility in polar solvents (83.05 g/100 mL of water at 20°C) and hygroscopic properties. It functions as an antimanic drug as well as a geroprotector. It is a lithium salt and an inorganic chloride.
Properties
Lithium chloride is a white hygroscopic solid that is soluble in water, alcohol, and ether. Lithium chloride has the chemical formula LiCl. It is formed when hydrochloric acid reacts with lithium hydroxide. The resulting solution is evaporated to produce a mixture of saturated solution and crystals of lithium chloride.
- Melting point: 605 °C (lit.)
- Boiling point: 1383 °C/1 atm (lit.)
- Density: 2.06
- Vapor pressure: 1.33 hPa (547 °C)
- Flash point: -4 °F
- Storage temp.: 2-8°C
- Solubility H2O: soluble
- Form: beads
- Color: White to gray
- Specific Gravity: 2.068
- Odor: Odorless

Chemical properties
Unlike the other alkali metal chlorides, the salt forms crystalline hydrates. There are mono-, tri-, and pentahydrates. Heat the hydrates to regenerate the anhydrous salt. In addition, LiCl absorbs up to four equivalents of ammonia/mol. Lithium chloride solutions, like any other ionic chloride, can serve as a source of chloride ion, for example, by forming a precipitate when treated with silver nitrate:
LiCl + AgNO3 → AgCl + LiNO3
The Li+ ion acts as a weak Lewis acid under certain circumstances; for example, one mole of lithium chloride is capable of absorbing up to four moles of ammonia.
Preparation
Lithium chloride is created by combining lithium carbonate and hydrochloric acid. The hydrate is heated in a stream of hydrogen chloride to produce anhydrous LiCl.
Lithium chloride can be made by reacting lithium carbonate or lithium hydroxide with hydrochloric acid and then crystallizing it:
(1) Li2CO3 + 2HCl → 2LiCl + CO2 + H2O
(2) LiOH + HCl → LiCl + H2O
The anhydrous salt is produced by crystallization above 95°C. When a hot solution cools, it forms crystals of monohydrate, LiCl.H2O. The solid is separated from the solution, and the supernatant solution is recycled for further evaporation. Anhydrous lithium chloride is obtained by drying the crystals.
Lithium chloride can be made from its constituent elements by heating lithium metal with chlorine gas. It can also be obtained naturally from brine.
Lithium chloride is most easily produced by reacting lithium hydroxide or lithium carbonate with hydrochloric acid. It can also be made through a highly exothermic reaction of lithium metal with chlorine or anhydrous hydrogen chloride gas. Anhydrous LiCl is made from the hydrate by gently heating it in a hydrogen chloride atmosphere to prevent hydrolysis.
Uses
Lithium chloride is primarily used in the electrolysis of a LiCl/KCl melt at 450 °C (842 °F) to produce lithium metal. In automobile parts, LiCl is also used as a brazing flux for aluminum.
Lithium chloride is also used to produce dark red flames as a flame colorant. It is employed as a desiccant in the drying of air streams. Its desiccant properties can be used to produce potable water by absorbing moisture from the air and then releasing it when heated.
Lithium chloride is used in organic synthesis in more specialized applications, such as as an additive in the Stille reaction. It can also be used to precipitate RNA from cellular extracts in biochemical applications.