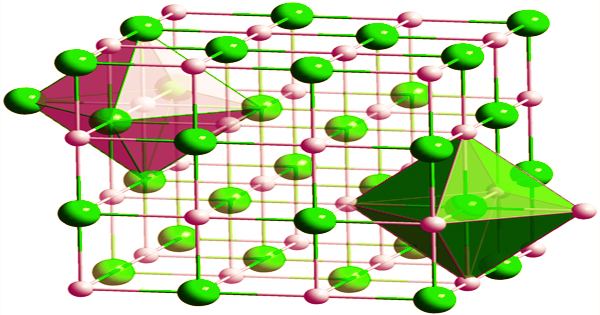
Lithium hydride is a crystalline mass or powder that is white or translucent. It’s a white solid with the formula LiH; cubic; r.d. 0.82; m.p. 680°C; decomposes at 850°C. It is made by combining the elements directly at temperatures above 500°C. Although commercial samples are grey, this alkali metal hydride is a colorless solid. It has a high melting point and is insoluble but reactive with all protic organic solvents, making it a salt-like (ionic) hydride. Owing to the presence of minute quantities of colloidally dispersed lithium, the commercial product is light bluish-gray lumps.
The fact that hydrogen is released from the anode during electrolysis of the molten salt supports the theory that the bonding in lithium hydride is mainly ionic, i.e., Li+H-. Certain molten salts, such as lithium fluoride, lithium borohydride, and sodium hydride, are soluble and nonreactive with lithium hydride. It is the lightest ionic compound, with a molecular mass of slightly less than 8.0. The majority of lithium hydride is used as a raw material in the manufacture of lithium aluminum hydride and lithium amide, which are also manufactured in large amounts.
Hydrogen and lithium hydroxide are generated when the compound reacts violently and exothermically with water. It’s used to make other hydrides as a reducing agent, and the 2Hisotopic compound lithium deuteride is especially useful for deuterating a variety of organic compounds. LiH is a diamagnetic and an ionic conductor with a conductivity gradually increasing from 2×10−5 Ω−1cm−1 at 443 °C to 0.18 Ω−1cm−1 at 754 °C; there is no break in this rise as it approaches the melting point. Lithium hydride has also been used as a thermal neutron shielding material.
It is used as a transportable hydrogen source because of its high hydrogen content, for example, in inflatable rubber dinghies and balloons. The dielectric constant of LiH decreases from 13.0 (static, low frequencies) to 3.6 (high frequencies) (visible-light frequencies). LiH has a Mohs hardness of 3.5, making it a soft material. Its compressive creep (per 100 hours) rapidly increases from < 1% at 350 °C to > 100% at 475 °C, meaning that LiH can’t provide mechanical support when heated. Furthermore, LiH’s high fusion heat combined with its light weight make it an ideal heat storage medium for solar power plants on satellites, as well as a heat sink for a variety of applications. Typically, LiH is handled at temperatures above its melting point (680oC) during the production process.
The thermal conductivity of LiH decreases with temperature and morphology: at 50 °C, the corresponding values are 0.125 W/(cmK) for crystals and 0.0695 W/(cmK) for compacts, and at 500 °C, the corresponding values are 0.036 W/(cmK) for crystals and 0.0432 W/(cmK) for compacts. At room temperature, the linear thermal expansion coefficient is 4.2105/°C. With lithium cations and hydride anions, lithium hydride is a typical ionic hydride. As molten material is electrolyzed, lithium metal is formed at the cathode and hydrogen is formed at the anode. The release of hydrogen gas from the lithium hydride-water reaction is also representative of a negatively charged hydrogen.
Lithium hydride (LiH) is produced on a broad scale by combining elements at 700-900 degrees Celsius. It’s a transparent, odorless, off-white to grayish solid or white powder that darkens quickly when exposed to light. Lithium metal is treated with hydrogen gas to create it:
2 Li + H2 → 2 LiH
At temperatures above 600 °C, this reaction is particularly fast. At a 2-hour residence period, adding 0.001–0.003 percent biomass, or rising temperature or pressure, increases the yield up to 98 percent. With the evolution of hydrogen, lithium hydride reacts vigorously with water to form lithium hydroxide:
LiH + H2O → LiOH + H2
The hydride also reacts with ammonia forming lithium amide and evolving hydrogen:
LiH + NH3 → LiNH2 + H2
Since lithium hydride is a powerful reducing agent, it will react with oxygen-containing compounds. Many highly stable metal and nonmetal oxides can be reduced. Thermal decomposition of lithium aluminum hydride (200 °C), lithium borohydride (300 °C), n-butyllithium (150 °C), or ethyllithium (120 °C), as well as several reactions involving lithium compounds with low stability and usable hydrogen content, are less popular methods of LiH synthesis.
The most common industrial application is the manufacture of high purity silane from silicon halides reacting in a lithium chloride-potassium chloride eutectic. LiH is produced as a lumped powder that can be compressed into pellets without the use of a binder in chemical reactions. Casting from the melt allows for more complex shapes. As lithium hydride reacts with aluminum hydride, lithium aluminum hydride is formed, which is a strong reducing agent.:
LiH + AlH3 → LiAlH4
Lithium hydride, which is made up of Li+ and H– ions, has the properties of both cationic and anionic ionic salts, as well as being a solid electrolyte. It dissociates into Li+ and H¯ ions when electrolyzed at temperatures slightly below its melting point. At the anode, hydrogen gas is liberated. Ball milling lithium metal under high hydrogen pressure is a more energy efficient way to make lithium hydride powder. The high ductility of lithium metal makes cold welding a problem with this process. Cold welding can be avoided by using small quantities of lithium hydride powder.
Lithium hydride is used to make lithium aluminum hydride and silane, as well as being an effective reducing agent, a condensation agent in organic synthesis, a portable hydrogen source, and a lightweight nuclear shielding material. It’s now being used in space power systems to store thermal energy. LiH powder reacts quickly with low-humidity air to produce LiOH, Li2O, and Li2CO3. The powder spontaneously ignites in moist air, creating a mixture of products that includes nitrogenous compounds. When the lump material mixes with humid air, a viscous substance forms on the surface.
This substance is relatively poisonous to humans; inflammation of the skin and mucous membrane tissues is more likely than death. The ignition temperature is influenced by the surface condition of LiH, the presence of oxides on the metal dish, and other factors. Dry oxygen does not react with crystalline LiH until it is heated to a high temperature, at which point it produces almost explosive combustion. Lithium hydride is a bluish-white crystal that catches fire when exposed to moisture. It’s also used to make hydrogen gas, which is released when LiH gets wet. LiH is an excellent desiccant and reducing agent, as well as a radiation shield produced by nuclear reactions.
Information Sources: