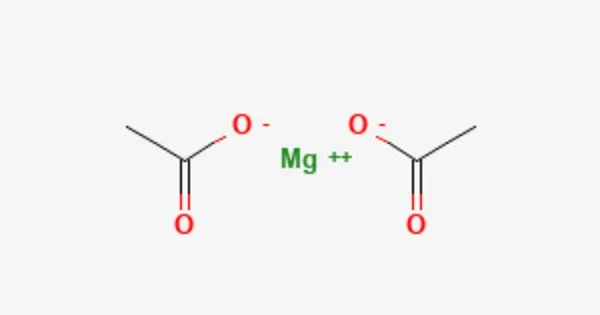
Anhydrous magnesium acetate has the chemical formula Mg(C2H3O2)2, and magnesium acetate tetrahydrate has the chemical formula Mg(CH3COO)2 • 4H2O. It is a chemical compound made up of magnesium, carbon, hydrogen, and oxygen.
The oxidation state of magnesium in this chemical is 2+. It is the anhydrous (water-free) form of magnesium acetate. Magnesium acetate is the magnesium salt of acetic acid. It is deliquescent and decomposes into magnesium oxide when heated. Magnesium acetate is a common source of magnesium in biological reactions.
Properties
Magnesium acetate appears as white hygroscopic crystals. It smells like acetic acid and is soluble in water. When it is in an aqueous solution its pH will be on the alkaline side of neutral. It is typically appears as a white crystalline powder. It is relatively stable at room temperature but can absorb moisture from the air, forming hydrated magnesium acetate over time.
- Chemical formula: Mg(CH3COO)2
- Molar mass: 142.394 (anhydrous) 214.455 (tetrahydrate)
- Appearance: White hygroscopic crystals
- Density: 1.45 g/cm3 (tetrahydrate)
- Melting point: 80 °C (176 °F; 353 K) (tetrahydrate)
- Solubility in water: Soluble
- Magnetic susceptibility (χ): −116.0·10−6 cm3/mol (+4 H2O
Storage
Because it is highly hygroscopic, it must be stored away from water. In addition, it is incompatible with powerful oxidizers and should not be used with them. It is a salt generated when magnesium oxide or magnesium hydroxide reacts with acetic acid. In chemical processes, it provides both magnesium and acetate ions.
Uses
- Deicing Agent: It is sometimes used as a deicing agent to prevent the formation of ice on roads and sidewalks during winter. When spread on icy surfaces, it lowers the freezing point of water, helping to melt ice.
- Catalyst: It can be used as a catalyst in certain chemical reactions, particularly in organic synthesis.
- Desiccant: Due to its ability to absorb moisture, anhydrous magnesium acetate can serve as a desiccant or drying agent in laboratories and industrial processes.
- Fire Retardant: In some cases, it is used as a fire retardant in various applications.
Safety
Magnesium acetate is a generally safe substance to handle, with a health hazard rating of zero. However, it should always be handled while wearing gloves and protective goggles. If it gets into the eyes, on the skin, or is consumed or inhaled, it will cause irritation in the following areas: eyes, skin, gastrointestinal system, and lungs.