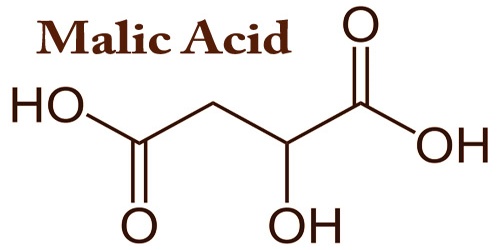
Malic acid, also known as hydroxysuccinic acid, is an organic compound (colorless solid) with the molecular formula C4H6O5. This is a 2-hydroxydicarboxylic acid which is succinic acid, in which a hydroxy group replaces one of the hydrogens attached to a carbon. It is a white or almost white, crystalline or granulated powder with a faint odor and a highly acidic taste. Malic acid is a dicarboxylic acid produced by all living organisms which contribute to the sour fruit taste and is used as a food additive. It has two stereoisomeric forms (L- and D-enantiomers), though only the L-isomer exists naturally.
Malic acid could be a 2-hydroxydicarboxylic acid and a C4-dicarboxylic acid; it derives from a carboxylic acid. It’s a conjugate acid of a malate(2-) and a malate. Malic acid exists in two optically active forms and a racemic mixture. It’s employed in medicine and located in apples and other fruits. The salts and esters of malic acid are called malates; the malate anion is an intermediate within the acid cycle.
The word ‘malic’ derives from the Latin ‘mālum’, meaning ‘apple’; it is also the name of the genus Malus, which includes both apples and crabapples; and the basis of other taxonomic classifications like Maloideae, Malinae, and Maleae. The naturally occurring isomer is the L-form that was used in apples and many other plants and fruits. Selective α-amino antioxidant reagent to derivatives of amino acid. Versatile synthon for the preparation of chiral compounds including κ-opioid rece.
Malic acid is the dominant acid in many fruits including apricots, blackberries, blueberries, cherries, grapes, mirabelles, peaches, pears, prunes, and quince and is found in lower amounts in other fruits, such as citrus fruits. In its ionized form its malate, an intermediate of the TCA cycle together with fumarate. It may be formed from pyruvate together of the anaplerotic reactions. Malic acid is formed in the presence of suitable catalysts, by hydrating maleic and fumaric acids. The formed malic acid is then removed from the mixture of the equilibrium drug.
Actually, malic acid may be a colorless crystalline acid that’s found in unripe fruits. As compared to acid, it’s slightly less soluble but continues to be readily soluble in water with a solubility of 132 g/100 ml at 20°c. It begins losing water very slowly at temperatures above 150 ° c to yield acid. It is used for soft beverages, dry-mix beverages, puddings, jellies, and fruit filling. Malic acid, when added to food products, is denoted by E number E296; it provides 10 kJ (2.39 kilocalories) of energy per gram during digestion.
Moreover, malic acid possesses chelating and antioxidant properties. It can be used as a synergist with butylated hydroxytoluene to retard oxidation in vegetable oils. Malic acid was important within the discovery of the Walden inversion and also the Walden cycle, within which (−)-malic acid first is converted into (+)-chlorosuccinic acid by the action of phosphorus pentachloride. Wet silver oxide then converts the chlorine compound to (+)-malic acid, which then reacts with PCl5 to the (−)-chlorosuccinic acid. The cycle is completed when silver oxide takes this compound back to (−)-malic acid. Therapeutically, malic acid has been used topically together with benzoic acid and salicylic acid to treat burns, ulcers, and wounds.
Information Sources: