Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalyzes, such as hydroformylation and Reppe chemistry. They are volatile and low-melting compounds of the Mx(CO)y type that decompose on heating into carbon monoxide and metal, their properties being conditioned by the structure of the carbonyl molecules and the type of chemical bonds in them. In general, the metal carbonyls are produced by the direct action of carbon monoxide on the finely divided metal.
Metal Carbonyls are important classes of organometallic compounds that have been studied for a long time. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. They are used in the preparation of metals of exceptionally high purity and as catalysts in organic syntheses. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes.
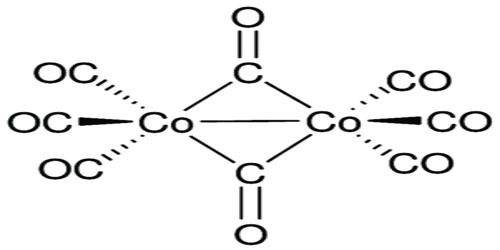
Structure of Metal Carbonyls:
- Due to the donation of electrons by the carbonyl molecules to the vacant orbitals of the metal, a metal-carbon σ bond is formed.
- Due to the donation of a pair of electrons from a filled d orbital metal into the vacant antibonding π* orbital of carbonyl ligand, a metal-carbon π bond is formed.
In a metal carbonyl, the metal-carbon bond has the characteristics of both σ and π bonds. The metal-carbon bonds in metal carbonyls have both σ and π characters. A σ bond is formed when the carbonyl carbon donates a lone pair of electrons to the vacant orbital of the metal. A π bond is formed by the donation of a pair of electrons from the filled metal d orbital into the vacant anti-bonding π orbital. The σ bond strengthens the π bond and vice-versa. Thus, a synergic effect is created due to this metal-ligand bonding. The bond between the carbonyl molecule and the metal becomes stronger by the synergic effect that the metal-ligand bond produces.
Metal carbonyls are toxic by skin contact, inhalation, or ingestion, in part because of their ability to carbonylate hemoglobin to give carboxyhemoglobin, which prevents the binding of O2. The electronic structures of most metal carbonyls containing one metal atom per molecule (mononuclear carbonyls) bear striking resemblances to those of the noble-gas elements. For example, tetracarbonylnickel has 36 electrons surrounding the nickel nucleus.