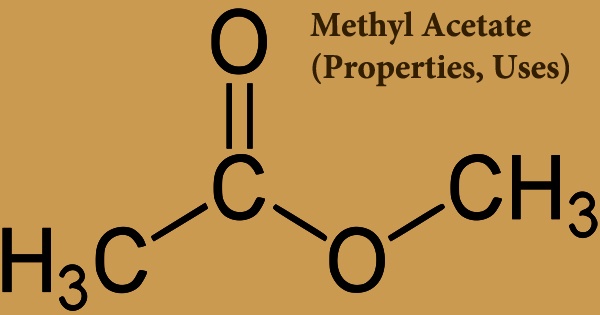
Methyl acetate, also known as MeOAc, acetic acid methyl ester, or methyl ethanoate, is an acetate ester formed when acetic acid and methanol are formalized. It is a carboxylate ester with the formula CH3COOCH3; it’s a flammable liquid with a nice odor that reminiscent of certain glues and nail polish removers. Since it is weakly polar and lipophilic, methyl acetate is sometimes used as a solvent, although its close relative, ethyl acetate, is a more common solvent since it is less toxic and less water-soluble. It’s a colorless, flammable liquid with a low boiling point (57 °C) that’s used as a solvent for a variety of resins and oils.
Methyl acetate is a flammable substance with a good aroma that reminds some people of glues and nail polish removers. At room temperature, it has a solubility of 25% in water. It has a much greater solubility in water at higher temperatures. In the presence of heavy aqueous bases or acids, methyl acetate is unstable. It acts as a polar aprotic solvent, a scent, and an EC 3.4.19.3 (pyroglutamyl-peptidase I) inhibitor. It’s a volatile organic compound that’s an acetate ester, a methyl ester, and an acetate ester.
As a byproduct of the manufacture of acetic acid, methyl acetate is generated industrially by carbonylation of methanol. With a flashpoint of -10° C and a flammability level of 3, it is extremely flammable. It is highly miscible with all typical organic solvents (alcohols, ketones, glycols, and esters), but only has a minor miscibility in water, which becomes more soluble at higher temperatures. Apples, oranges, and bananas are among the fruits that contain it. Methyl acetate can also be made by esterifying acetic acid with methanol in the presence of strong acids like sulfuric acid; this process is well-known thanks to Eastman Kodak’s enhanced method of reactive distillation.
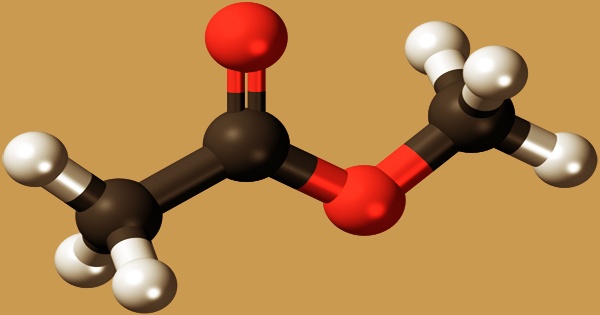
The odor of methyl acetate is good and fruity, and the taste is slightly bitter. It can be made by boiling acetic acid and methanol in the presence of acid catalysts, or by heating methanol under pressure with excess carbon monoxide in the presence of a catalyst. Methyl acetate can be made in a variety of ways. Carbonylation is one method that is used in industry. Carbon monoxide substrates are brought together in these reactions. Methanol is heated with acetic acid in the presence of sulfuric acid to make methyl acetate.
The esterification of methanol and acetic acid in the presence of a strong acid is another form of processing. This reaction also employs the use of sulfuric acid as a catalyst. It is hydrolyzed back into methanol and acetic acid in the presence of strong bases such as sodium hydroxide or strong acids such as hydrochloric acid or sulfuric acid, particularly at high temperatures. Methyl acetate found in apple, banana, sweet and sour cherry, tangerine juice, black currants, guava, grapes, melon, peach, pear, pineapple, strawberry, cabbage, tomato, clove bud, peppermint oil, vinegar, bread, cheeses, butter, yogurt, beef, beer, cognac, rum, whiskies, cider, sherry, grape wines, cocoa, coffee, filbert, peanut, honey, soybean, olive, passion fruit, fruit brandies, fig, gin, kiwifruit, clary sage, arrack and nectarine.
With respect to the ester, the conversion of methyl acetate back into its constituents by an acid is a first-order reaction. With regard to all reactants, the reaction of methyl acetate and a substrate, such as sodium hydroxide, is a second-order reaction.
As a result, only methanol and acetic acid were present in the blood and urine, not methyl acetate. When methyl acetate is ignited, it produces thick, irritating, and poisonous fumes that can travel long distances. These vapors are also explosive, and if they are able to return to the source of ignition, they risk bursting. A Lewis base, methyl acetate, forms 1:1 adducts with a number of Lewis acids. With EB =1.63 and CB = 0.95, it is graded as a hard base and is a base in the ECW model.
Methyl acetate is used as a solvent for lacquers, resins, oils, and nitrocellulose, as well as in paint removers, flavoring agents, and artificial leather production. Methyl acetate is commonly used as a low-toxicity volatile solvent in glues, paints, and nail polish removers. Carbonylation of methyl acetate produces acetic anhydride, a method inspired by Monsanto’s acetic acid synthesis. The reactivity of methyl acetate is similar to that of other compounds in the ester group. It is converted back to methanol and acetic acid in the presence of heavy bases or acids such as sodium hydroxide, hydrochloric acid, and sulfuric acid at high temperatures.
Methyl acetate is highly soluble in water; it tends to be quickly degraded by soil and water microorganisms; and it does not accumulate in aquatic organisms. Headaches, fatigue, and drowsiness are caused by inhalation; high concentrations can cause central nervous system depression and optic nerve damage. Liquid irritates the eyes and can lead to skin defatting and cracking. Ingestion induces headaches, dizziness, drowsiness, and fatigue, as well as the potential for serious eye injury. Methyl acetate should be contained in a flammable liquid storage environment, securely sealed in drummed containers such as stainless steel, aluminum, or carbon steel isotanks. This space should be kept cool, dry, and well-ventilated, away from direct sunlight, fire, open flames, and strong acids, alkalis, nitrates, and strong oxidizers.
In laboratory animals, methyl acetate has not been tested for cancer, developmental, or reproductive effects. The United States Environmental Protection Agency’s IRIS program, the International Agency for Research on Cancer, and the United States National Toxicology Program’s 12th Report on Carcinogens have not measured the potential for methyl acetate to cause cancer in humans. Isolate the leak for at least 50 meters in all directions if methyl ethanoate is spilled. Because of the low flashpoint of methyl acetate, water extinguishers may be ineffective in the event of a fire. Alcohol-resistant foam extinguishers are ideal for both small and large fires. Methyl ethanoate is also used as a chemical intermediate for the synthesis of chlorophacinone, diphacinone, fenfluramine, o-methoxyphenylacetone, p-methoxyphenylacetone, methyl cinnamate, methyl cyanoacetate, methyldopa, and phenylacetone and in the manufacturing of cellulose adhesives and perfumes.
By heating methanol with an excess of carbon monoxide under pressure in the presence of a catalyst, or by boiling acetic acid and methanol in the presence of acid catalysts. Methyl ethanoate is used as a flavoring agent in food additives for rum, brandy, and whiskey, as well as in adhesives, cleaning materials, personal care, and cosmetic products, lubricants, fast-drying paints like lacquers, motor vehicle coatings, furniture coatings, automotive coatings (low boiling point), inks, resins, oils, and electronic products. The paint, coatings, cosmetics, textiles, and automotive industries are the primary end markets for this product.
Information Sources: