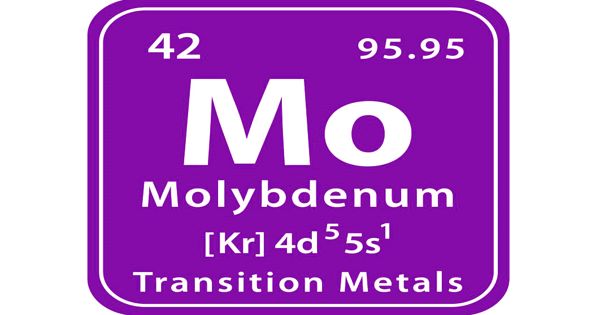
Molybdenum is a chemical element with the symbol Mo and atomic number 42. It is a silvery-white metal that is ductile and highly resistant to corrosion. It has one of the highest melting points of all pure elements. Molybdenum is attacked slowly by acids. It more commonly occurs as a dark gray or black powder with a metallic luster.
Molybdenum minerals have been known throughout history, but the element was discovered (in the sense of differentiating it as a new entity from the mineral salts of other metals) in 1778 by Carl Wilhelm Scheele. The metal was first isolated in 1781 by Peter Jacob Hjelm.
Molybdenum is a silvery-white metal that is ductile and highly resistant to corrosion. It has one of the lowest coefficients of thermal expansion among commercially used metals.
Physical properties
In its pure form, molybdenum is a silvery-grey metal with a Mohs hardness of 5.5 and a standard atomic weight of 95.95 g/mol. It has a high elastic modulus, and only tungsten and tantalum, of the more readily available metals, have higher melting points. It does not occur naturally in the pure metallic form. It is principally found as an oxide or sulfide compounds.
- atomic number: 42
- atomic weight: 95.94
- melting point: 2,610 °C (4,730 °F)
- boiling point: 5,560 °C (10,040 °F)
- specific gravity: 10.2 at 20 °C (68 °F)
- oxidation states: 0, +2, +3, +4, +5, +6.

It has one of the highest melting points of all pure elements — only the elements tantalum and tungsten have higher melting points. It has a melting point of 2,623 °C (4,753 °F); of the naturally occurring elements, only tantalum, osmium, rhenium, tungsten, and carbon have higher melting points. It has one of the lowest coefficients of thermal expansion among commercially used metals.
Occurrences
Molybdenum does not occur naturally as a free metal on Earth; it is found only in various oxidation states in minerals. It has one of the highest melting points of all pure elements — only the elements tantalum and tungsten have higher melting points.
The free element, a silvery metal with a gray cast, has the sixth-highest melting point of any element. It readily forms hard, stable carbides in alloys, and for this reason, most of the world production of the element (about 80%) is used in steel alloys, including high-strength alloys and superalloys.
Uses
Molybdenum improves the strength, toughness, resistance to wear and corrosion, and ability to harden steel. It has one of the highest melting points of all pure elements – only the elements tantalum and tungsten have higher melting points.