Nano-thermite – a metastable intermolecular composite
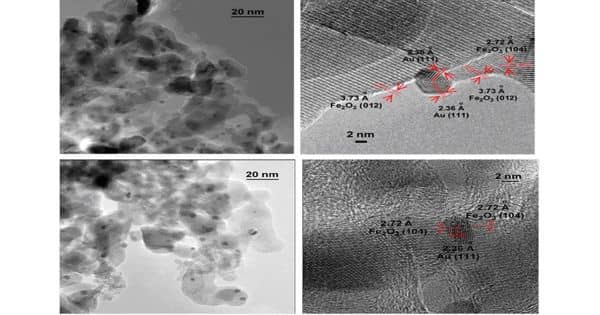
Nanothermites are the most important family of energetic materials in contemporary pyrotechnics. It is also known as super-thermite, is a metastable intermolecular composite (MICs) characterized by a particle size of its main constituents, a metal, and a metal oxide, under 100 nanometers. This allows for high and customizable reaction rates. The nano-thermite composites show consistency in onset temperature even with different ratios of Al and Fe2O3, which suggests uniform interfacial formation, where the nano-thermite reactions are initiated.
Nano-thermites contain an oxidizer and a reducing agent, which are intimately mixed on the nanometer scale. They are combustible materials made up of inorganic fuel and an oxidizer.
Thermite is a mixture of aluminum powder and metal oxide (such as iron oxide) that when ignited evolves a great deal of heat and is used in welding and in incendiary bombs. Nano-thermites contain an oxidizer and a reducing agent, which are intimately mixed on the nanometer scale. MICs, including nano-thermitic materials, are a type of reactive materials investigated for military use, as well as for general applications involving propellants, explosives, and pyrotechnics. Thermite reactions describe the reaction between oxidizer and fuel, which involves a reduction of the metallic oxidizer and oxidation of the fuel. The fuels can be aluminum, magnesium, titanium, zinc, silicon, or boron. Because of its high volumetric energy density and affinity for oxygen, aluminum is used widely as the fuel component.
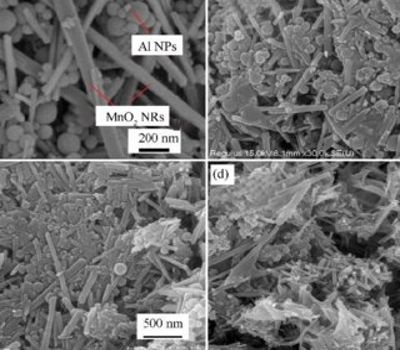
Thermal and combustion behavior of Al-MnO2 nanothermite
What distinguishes MICs from traditional thermites is that the oxidizer and a reducing agent, normally iron oxide and aluminum, are in the form of extremely fine powders (nanoparticles). Nanothermite objects must have both high open porosity and good mechanical strength. Nanothermite foams meet both of these criteria, and they are prepared by dispersing a nano-sized aluminothermic mixture (Al/WO3) in orthophosphoric acid.
Nanothermites are combustible materials made up of inorganic fuel and an oxidizer. In nano-thermite foams, aluminum is the fuel, and aluminum phosphate and tungsten trioxide are the oxidizing moieties. This dramatically increases the reactivity relative to micrometer-sized powder thermite. As the mass transport mechanisms that slow down the burning rates of traditional thermites are not so important at these scales, the reaction proceeds much more quickly. Even with significant efforts to improve traditional thermite composites, the performance of low reaction rates and long ignition delays cannot meet the demands of microscale applications. To improve the performance of thermite systems, researchers must develop new systems using particles of a different dimension