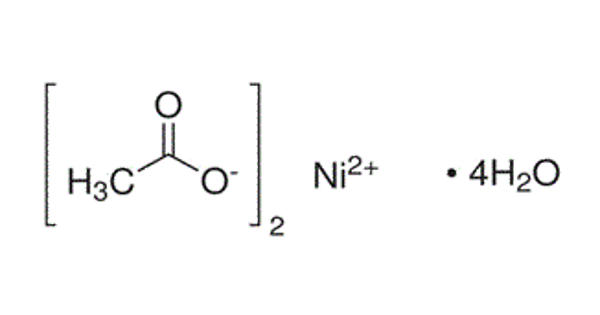
Nickel acetate is a moderately water-soluble chemical compound. It is the name for the coordination compounds with the formula Ni(CH3CO2)2·x H2O where x can be 0, 2, and 4. This compound can be obtained by treating nickel carbonate with acetic acid. The green tetrahydrate Ni(CH3CO2)2·4 H2O is most common. It is used for electroplating.
Properties
The nickel acetate crystals have been seen to adopt an octahedral structure with nickel at the centre coordinated by four water molecule and two acetate ligands. It is a green, crystalline, water-soluble solid, used chiefly in nickel-plating.
- Appearance: Green Solid
- Molecular Weight: 248.841 g/mol
- Density : 1.78 g/cm3
- Melting Point : Decomposes on heating
- Solubility In Water: Soluble

Synthesis and structure
Nickel acetate solution is an inorganic compound of nickel and acetic acid. The highest quality raw materials in the production of Nickel Acetate are used. End users include electroplating and sealing agent for anodized aluminium.
The compound can be prepared by treating nickel or nickel(II) carbonate with acetic acid:
NiCO3 + 2 CH3CO2H + 3 H2O → Ni(CH3CO2)2·4 H2O + CO2
The green tetrahydrate has been shown by X-ray crystallography to adopt an octahedral structure, the central nickel centre being coordinated by four water molecules and two acetate ligands. It may be dehydrated in vacuo, by reaction with acetic anhydride, or by heat.
Uses
- Used as a mordant in the textile industry
- It is used in textile dyeing processes, in electroplating, and as a catalyst intermediate.
- Used in anodizing applications
Safety
Nickel salts are carcinogenic and irritate the skin. Symptoms of overexposure to nickel can cause sensitization, dermatitis, allergic asthma and pneumonitis. Exposure to this substance can cause severe dermatitis, skin and asthma-like allergies and causes damage to the lungs, kidneys, gastrointestinal tract and neurological system.
Information Source: