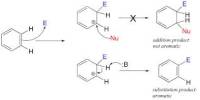
Nitrogen is the chemical element with the symbol N and atomic number 7. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.09 percent of Earth’s atmosphere by volume. It is the lightest member of group 15 of the periodic table, often called the pnictogens. Nitrogen changes from a gas into a liquid at a temperature of -195.79°C (-320.42°F). It changes from a liquid to a solid at a temperature of -210.01°C (-346.02°F). It is a common element in the universe, estimated at about seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bind to form dinitrogen, a colorless and odorless diatomic gas with the formula N2. It is slightly soluble in water. About two liters of nitrogen can be dissolved in 100 liters of water.
- atomic number: 7
- atomic weight: 14.0067
- melting point: −209.86 °C (−345.8 °F)
- boiling point: −195.8 °C (−320.4 °F)
- density: (1 atm, 0° C) 1.2506 grams/litre
- usual oxidation states: −3, +3, +5
It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772. Although Carl Wilhelm Scheele and Henry Cavendish had independently done so at about the same time, Rutherford is generally accorded the credit because his work was published first. The name nitrogène was suggested by French chemist Jean-Antoine-Claude Chaptal in 1790 when it was found that nitrogen was present in nitric acid and nitrates.
Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids (DNA and RNA) and in the energy transfer molecule adenosine triphosphate. It is an essential element for life, because it is a constituent of DNA and, as such, is part of the genetic code. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The atmosphere of Earth consists of 75.51 percent by weight (or 78.09 percent by volume) of nitrogen; this is the principal source of nitrogen for commerce and industry. India, Russia, the United States, Trinidad and Tobago, and Ukraine were the top five producers of nitrogen (in the form of ammonia) in the early 21st century.
Uses
Nitrogen is important to the chemical industry. It is used to make fertilizers, nitric acid, nylon, dyes, and explosives. The largest use of nitrogen is for the production of ammonia (NH3). Nitrogen gas is largely inert and is used as a protective shield in the semiconductor industry and during certain types of welding and soldering operations. This gas has a variety of applications, including serving as an inert replacement for air where oxidation is undesirable. Liquid nitrogen is also used to cryogenically freeze objects. Nitrogen gas is also used to provide an unreactive atmosphere. It is used in this way to preserve foods, and in the electronics industry during the production of transistors and diodes.