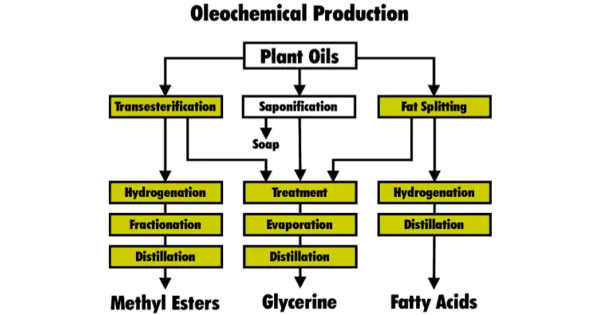
The study of vegetable oils, animal oils and fats, and the oleochemicals derived from these fats and oils is known as oleochemistry. The resulting product is known as oleochemicals. They are chemical compounds derived from natural fats and oils that can be used as raw materials or additives in a variety of industries. Oleochemicals are created through a variety of chemical processes, including water-based hydrolysis, alcohol-based alcoholysis, and hydrogenation, to name a few.
Soap is the main product of this industry, with approximately 8.9×106 tons produced in 1990. Fatty acids, fatty acid methyl esters, fatty alcohols, and fatty amines are also important oleochemicals. All of these processes produce glycerol as a byproduct. Alcohol ethoxylates, alcohol sulfates, alcohol ether sulfates, quaternary ammonium salts, monoacylglycerols (MAG), diacylglycerols (DAG), structured triacylglycerols (TAG), sugar esters, and other oleochemical products are produced from these basic oleochemical substances.
As crude oil prices rose in the late 1970s, manufacturers shifted from petrochemicals to oleochemicals because plant-based lauric oils derived from palm kernel oil were less expensive. Since then, palm kernel oil has primarily been used in the manufacture of laundry detergent and personal care products such as toothpaste, soap bars, shower cream, and shampoo.
Processes
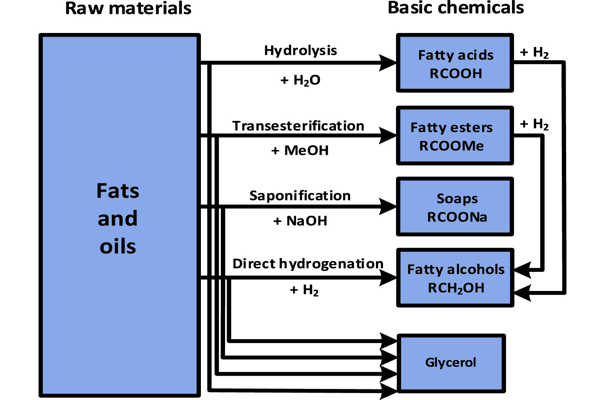
Important processes in oleochemical manufacturing include hydrolysis and transesterification, among others.
- Hydrolysis
The splitting (or hydrolysis) of the triglycerides produces fatty acids and glycerol follows this equation:
RCO2CH2–CHO2CR–CH2O2CR + 3 H2O → 3 RCOOH + HOCH2–CHOH–CH2OH
To this end, hydrolysis is conducted in water at 250°C. The cleavage of triglycerides with base proceeds more quickly than hydrolysis, the process being saponification. Saponification however produces soap, whereas the desired product of hydrolysis is the fatty acids.
- Transesterification
Fats react with alcohols (R’OH) instead of with water in hydrolysis) in a process called transesterification. Glycerol is produced together with the fatty acid esters. Most typically, the reaction entails the use of methanol (MeOH) to give fatty acid methyl esters:
RCO2CH2–CHO2CR–CH2O2CR + 3 MeOH → 3 RCO2Me + HOCH2–CHOH–CH2OH
FAMEs are less viscous than the precursor fats and can be purified to give the individual fatty acid esters, e.g. methyl oleate vs methyl palmitate.
- Hydrogenation
Because fatty acids and fatty esters are susceptible to hydrogenation, unsaturated fatty acids are converted into saturated fatty acids. Acids and esters can also be converted to fatty alcohols. Fatty acids are converted to fatty nitriles for some applications.
- Gelation
Liquid oil can also be immobilized in a 3D network provided by various molecules called oleogelators.
Applications
Making soaps and detergents is the most common application for oleochemicals, accounting for approximately 30% of the market share for fatty acids and 55% for fatty alcohols. Lauric acid is a chemical that is used to make sodium lauryl sulfate and related compounds, which are then used to make soaps and other personal care products.
Consumption of oleochemicals in the form of fatty acids, which are used to make soaps and detergents, surfactants, lubricants, varnishes, and pharmaceuticals, is also expected to drive market growth in the coming years.
Other uses for oleochemicals include the manufacture of lubricants, solvents, biodiesel, and bioplastics. Because of their use in biodiesel production, methyl esters have been the fastest growing sub-sector of oleochemical production in recent years.