In chemistry, an open-chain compound or acyclic compound is a compound with a linear structure, rather than a cyclic one. They are those in which carbon atoms are linked to each other either in linear or branched fashion such that the molecule is having open-chain structure. It is a series of atoms linked in a chain not joined together at its ends, and so represented in its structural formula. This chain may either be straight-chain or a branched-chain.
An open-chain compound having no side chains is called a straight-chain compound (also spelled as straight-chain compound). Examples: CH3 – CH3 – CH3 propane. Many of the simple molecules of organic chemistry, such as the alkanes and alkenes, have both linear and ring isomers, that is, both acyclic and cyclic, with the latter often classified as aromatic. These are an arrangement of atoms that do not form a ring, as in silicon compounds and various carbon compounds, such as aliphatic hydrocarbons. For those with 4 or more carbons, the linear forms can have straight-chain or branched-chain isomers. The lowercase prefix n- denotes the straight-chain isomer; for example, n-butane is straight-chain butane, whereas i-butane is isobutane. Aliphatic compounds, such as ethane and isopropyl alcohol, are open-chain compounds. Cycloalkanes are isomers of alkenes, not of alkanes, because the ring’s closure involves a C-C bond. It is convenient to distinguish between aliphatic and aromatic acids; the first-named being derived from open-chain hydrocarbons, the second from ringed hydrocarbon nuclei. Having no rings (aromatic or otherwise), all open-chain compounds are aliphatic. It is one of two broad classes of hydrocarbons, the other being aromatic compounds. These compounds retain their aliphatic nature and are best classified with open-chain compounds, into which, in general, they are readily converted. The most significant characteristic of aliphatic compounds is that most of them are flammable.
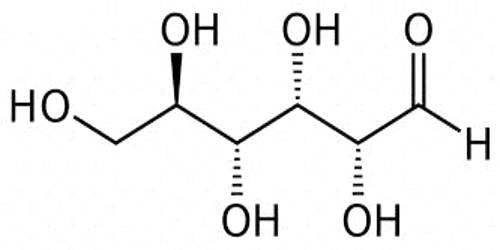
Typically in biochemistry, some isomers are more prevalent than others. Aliphatic or aliphatic acids are the acids of nonaromatic hydrocarbons. For example, in living organisms, the open-chain isomer of glucose usually exists only transiently, in small amounts; D-glucose is the usual isomer, and L-glucose is rare. Examples of aliphatic acids are butyric acid, propionic acid, and acetic acid.
Straight-chain molecules are often not literally straight, in the sense that their bond angles are often not 180°, but the name reflects that they are schematically straight. These compounds are also known as aliphatic compounds, they have branched or straight chains. For example, the straight-chain alkanes are wavy or “puckered”,