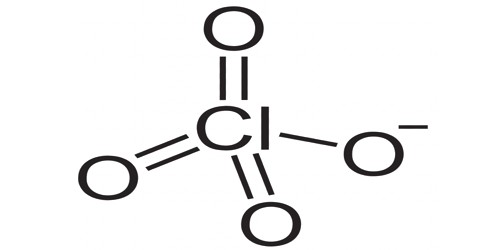
Perchlorate is an ion. Its chemical formula is ClO4-. It is a manufactured chemical and colorless salt that is most commonly used in rocket fuel. It contains chlorine in its +7 oxidation state. It is a negatively charged molecule made of one chlorine atom and four oxygen atoms. The majority of perchlorates are commercially produced salts. It occurs naturally in arid states in the Southwest United States, in nitrate fertilizer deposits in Chile, and in potash ore in the United States and Canada. It is a contaminant of emerging concern—,, especially in groundwater.
It is present in chemical compounds like ammonium perchlorate. It is a monovalent inorganic anion obtained by deprotonation of perchloric acid. It also forms naturally in the atmosphere. It is a strong oxidizing agent. It is a monovalent inorganic anion and a chlorine oxoanion. It is the anion resulting from the dissociation of perchloric acid and its salts upon their dissolution in water. It dissolves easily, is relatively stable and is mobile in water. It is a conjugate base of perchloric acid.
Perchlorate contamination in food, water, and other parts of the environment has been studied in the U.S. because of its harmful effects on human health. It reduces hormone production in the thyroid gland. It can disrupt the normal function of the thyroid gland in both children and adults. In fetuses and infants, thyroid hormones are critical for normal growth and development of the central nervous system.
Uses
- Perchlorate is used to control static electricity in food packaging. Sprayed onto containers it stops statically charged food from clinging to plastic or paper/cardboard surface.
- It is used in munitions, fireworks, explosives, airbag initiators for vehicles, matches, signal flares, fertilizers, chlorine cleaners, and pool chlorination chemicals.
- It may be found at sites associated with military and space rocket propellant use and development activities.