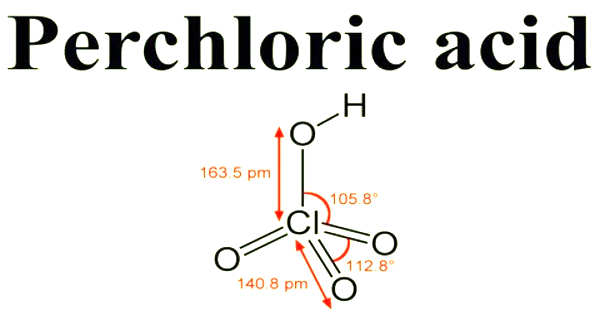
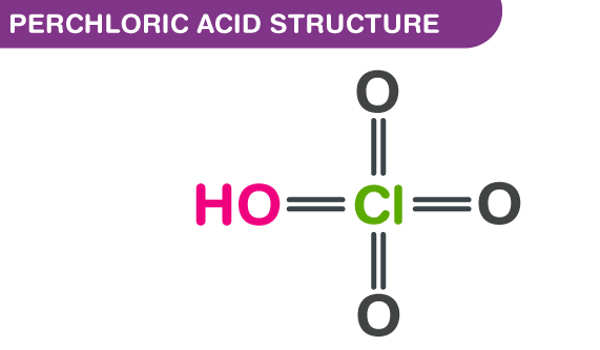
Perchloric acid is a colorless, odorless, and oily corrosive inorganic liquid. It has the formula HClO4 and is a mineral acid. This colorless compound, which is usually found as an aqueous solution, is a stronger acid than sulfuric acid and nitric acid. It has a boiling point of 203°C, a melting point of 19°C, and low vapor pressure of 6.8 mm Hg at 25°C. When heated, it is a powerful oxidizer, but aqueous solutions up to approximately 70% by weight at room temperature are generally safe, exhibiting only strong acid features and no oxidizing properties.
Perchloric acid is one of the strongest mineral acids and has a high oxidizing power at high temperatures, making it ideal for the digestion of matrices such as fat, proteins, and lipids because the resulting perchlorates are water-soluble.
Production
Perchloric acid is produced industrially by two routes. The traditional method exploits the high aqueous solubility of sodium perchlorate (209 g/100 mL of water at room temperature). Treatment of such solutions with hydrochloric acid gives perchloric acid, precipitating solid sodium chloride:
NaClO4 + HCl → NaCl + HClO4
The concentrated acid can be purified by distillation. The alternative route, which is more direct and avoids salts, entails anodic oxidation of aqueous chlorine at a platinum electrode.
Laboratory preparations
When barium perchlorate is treated with sulfuric acid, barium sulfate precipitates, leaving perchloric acid. It can also be made by boiling nitric acid and ammonium perchlorate while adding hydrochloric acid. Because of a concurrent reaction involving the ammonium ion, the reaction produces nitrous oxide and perchloric acid, which can be concentrated and purified significantly by boiling off the remaining nitric and hydrochloric acids.
Perchloric acid is commercially produced through either the reaction of alkali perchlorates with hydrochloric acid or the direct electrolytic oxidation of 0.5 N hydrochloric acid.
Properties
At room temperature, anhydrous perchloric acid is an unstable oily liquid. It forms at least five hydrates, several of which have been crystallographically characterized. These solids are made up of a perchlorate anion that is linked to H2O and H3O+ centers via hydrogen bonds. With water, perchloric acid forms an azeotrope that contains approximately 72.5 percent perchloric acid. This form of acid is commercially available and is indefinitely stable. These types of solutions are hygroscopic. Concentrated perchloric acid dilutes itself by absorbing water from the air if exposed to air.
Uses
Perchloric acid is useful for preparing perchlorate salts, especially ammonium perchlorate, an important rocket fuel component. It is used to separate potassium from sodium and in many laboratory tests and industrial processes. It is dangerously corrosive and readily forms potentially explosive mixtures. It is a strong acid used for complete digestions of organic material.
Information Source: