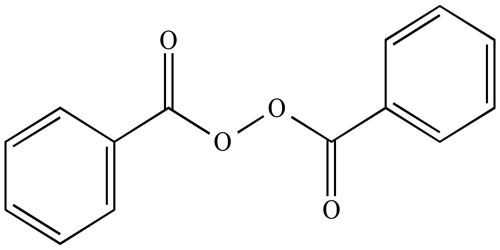
Peroxides are found in a number of excipients, including binders. The most common type of reactive peroxide moiety is organically bound peroxide (ROOH, where R is a carbon atom). They are a group of compounds with the structure R−O−O−R. The O−O group in peroxide is called the peroxide group or peroxo group. In contrast to oxide ions, the oxygen atoms in the peroxide ion have an oxidation state of −1. An organic peroxide is any organic (carbon-containing) compound having two oxygen atoms joined together (-O-O-). This chemical group is called a “peroxy” group.
Peroxides can react directly with oxidation-sensitive drugs and generate free radicals, which can initiate oxidative chain reactions. It is any of a class of chemical compounds in which two oxygen atoms are linked together by a single covalent bond. Several organic and inorganic peroxides are useful as bleaching agents, as initiators of polymerization reactions, and in the preparation of hydrogen peroxide and other oxygen compounds. Examples: hydrogen peroxide, di-tert-butyl peroxide, benzoyl peroxide, dicumyl peroxide, cumene hydroperoxide, tert-butyl hydroperoxide, phthaloyl peroxide, bis(methanesulfonyl) peroxide, see also: peroxy acids.
Two categories of peroxides exist in which one or both of the oxygen atoms are covalently linked to atoms other than hydrogen. The most common peroxide is hydrogen peroxide (H2O2), colloquially known simply as “peroxide”. It is marketed as a solution in water at various concentrations. Since hydrogen peroxide is nearly colorless, so are these solutions. It is mainly used as an oxidant and bleaching agent. One category is represented by cumene hydroperoxide, an organic compound used as a polymerization initiator and as a source of phenol and acetone, and peroxysulfuric acid, an inorganic compound used as an oxidizing agent. Applications of inorganic peroxides and peroxo compounds include household detergents, polymerization catalysts, effluent treatment, organic synthesis as well as uses in pyrotechnics, dough conditioning, hydrometallurgy and medicine.
However, hydrogen peroxide is also biochemically produced in the human body, largely as a result of a range of oxidase enzymes. Concentrated solutions are potentially dangerous when in contact with organic compounds. Aside from hydrogen peroxide, some other major classes of peroxides are:
- Peroxy acids, the peroxy derivatives of many familiar acids, examples being peroxymonosulfuric acid and peracetic acid.
- Metal peroxides, examples being barium peroxide (BaO2) and sodium peroxide (Na2O2).
- Organic peroxides, compounds with the linkage C−O−O−C or C−O−O−H. One example is tert-butylhydroperoxide.
- Main group peroxides, compounds with the linkage E−O−O−E (E = main group element), one example of which is potassium peroxydisulfate.