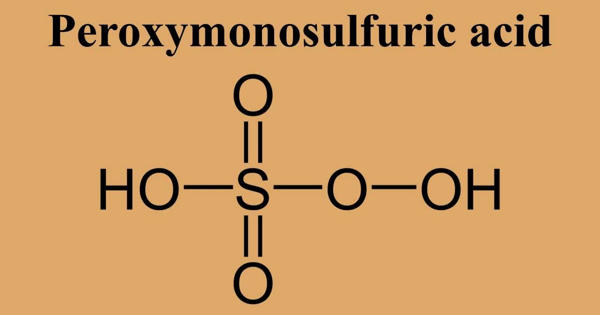
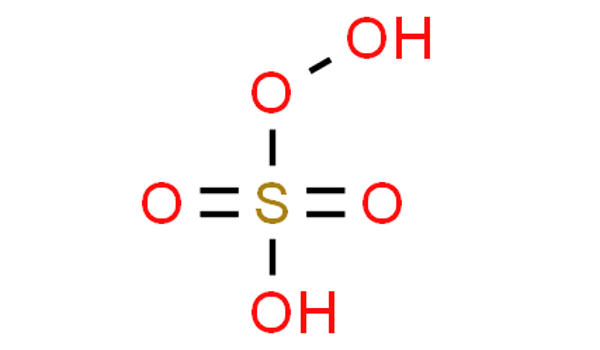
Peroxymonosulfuric acid, (H2SO5), is a sulfur oxoacid. It is also known as persulfuric acid, peroxysulfuric acid, or Caro’s acid. It is a conjugate acid of a peroxysulfate(1-). In this acid, the S(VI) center adopts its characteristic tetrahedral geometry; the connectivity is indicated by the formula HO–O–S(O)2–OH. It is one of the strongest oxidants known (E0 = +2.51 V) and is highly explosive.
H2SO5 is sometimes confused with H2S2O8, known as peroxydisulfuric acid. The disulfuric acid, which appears to be more widely used as its alkali metal salts, has the structure HO–S(O)2–O–O–S(O)2–OH.
Properties
- Chemical: Caro’s acid is among the strongest oxidizers known. It is unstable and is generally prepared within a few days of use. It is highly explosive, especially in mixtures with organic matter.
- Physical: Caro’s acid solutions are clear, colorless and are very viscous and have a oily consistency. The density is in the range 1.7 – 1.8 grams per milliliter.
Synthesis and production
The laboratory scale preparation of Caro’s acid involves the combination of chlorosulfuric acid and hydrogen peroxide.
H2O2 + ClSO2OH ⇌ H2SO5 + HCl
However, as the acid is unstable, it will rapidly degrade and become diluted.
Published patents include more than one reaction for preparation of Caro’s acid, usually as an intermediate for the production of potassium monopersulfate (PMPS), a bleaching and oxidizing agent. Purer Caro’s acid can be obtained by reacting very concentrated hydrogen peroxide with chlorosulfuric acid. One patent for production of Caro’s acid for this purpose gives the following reaction:
H2O2 + H2SO4 ⇌ H2SO5 + H2O
This is the reaction that produces the acid transiently in “piranha solution”.
Caro’s acid also may be prepared by adding sulfuric acid to aqueous slurries of sodium, ammonium or potassium persulfate.
Uses in industry
H2SO5 has been used for a variety of disinfectant and cleaning applications, e.g., swimming pool treatment and denture cleaning. Alkali metal salts of H2SO5 show promise for the delignification of wood. It is also used in laboratories as a last resort in removing organic materials since H2SO5 can fully oxidize any organic materials.
Ammonium, sodium, and potassium salts of H2SO5 are used in the plastic industry as polymerization initiators, etchants, desizing agents, soil conditioner, and for decolorizing and deodorizing oils.
Potassium peroxymonosulfate, KHSO5, is the potassium acid salt of peroxymonosulfuric acid. It is widely used as an oxidizing agent.
Hazards
Extremely corrosive. Pure Caro’s acid is highly explosive. Explosions have been reported at Brown University and Sun Oil. Highly irritating to skin, eyes, mucous membranes. As with all strong oxidizing agents, peroxysulfuric acid should be kept away from organic compounds such as ethers and ketones because of its ability to peroxidize these compounds, creating highly unstable molecules such as acetone peroxide.
Information Source: