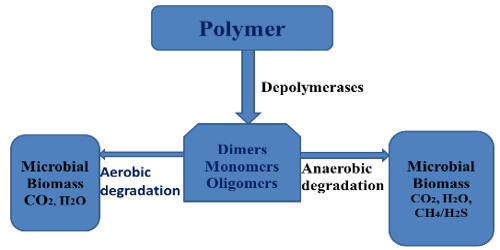
Polymer degradation is a change in the properties—tensile strength, color, shape, etc. of a polymer or polymer-based product under the influence of one or more environmental factors such as heat, light or chemicals such as acids, alkalis, and some salts. Degradation is often due to a change in the chemical and/or physical structure of the polymer chain, which in turn leads to a decrease in the molecular weight of the polymer. These changes are usually undesirable, such as cracking and chemical disintegration of products or, more rarely, desirable, as in biodegradation, or deliberately lowering the molecular weight of a polymer for recycling. Such changes occur primarily because of the effect of these factors on the chemical composition of the polymer. The changes in properties are often termed “aging”.
Polymer degradation includes all changes in both the chemical structure and physical properties of polymers or polymer-based products that lead to the loss of properties such as tensile strength, color, shape, etc., under the influence of processing conditions, or one or more environmental factors. In a finished product, such a change is to be prevented or delayed. The degradation of polymers to form smaller molecules may proceed by random scission or specific scission. Degradation can be useful for recycling/reusing polymer waste to prevent or reduce environmental pollution. The degradation of polyethylene occurs by random scission – a random breakage of the bonds within the polymer. Degradation can also be induced deliberately to assist in structure determination. As the recognition of polymer degradation improves, conservation guidelines are beginning to emerge.
All polymers will undergo some degradation during service life. The result will be a steady decline in their (mechanical) properties caused by changes to the molecular weight and molecular weight distribution and composition of the polymer. Other possible changes include:
- Embrittlement (chain hardening)
- Softening (chain scission)
- Color changes
- Cracking and charring (weight loss)
Polymer degradation is almost always faster in the presence of oxygen or air due to the accelerating reactions between oxygen and carbon-centered radicals (RO) released from the initial degradation products. Polymeric molecules are very large, and their unique and useful properties are mainly a result of their size. Degradation strongly influences the stability and durability of polymer materials, which can have dramatic consequences in the safety and reliability of products. Any loss in chain length lowers tensile strength and is a primary cause of premature cracking. It describes as a mixture of chemical processes and physical changes, occurring through the processing and storage resulting in the cleavage of main chain bonds.