Polyurethane is a plastic material, which exists in various forms. It is a polymer composed of organic units joined by carbamate (urethane) links. While most polyurethanes are thermosetting polymers that do not melt when heated, thermoplastic polyurethanes are also available. They are used in a diversity of products, from coatings and adhesives to shoe soles, mattresses, and foam insulation. The nature of the chemistry allows polyurethanes to be adapted to solve challenging problems, to be molded into unusual shapes, and to enhance industrial and consumer products by adding comfort, warmth, and convenience to our lives.
Polyurethanes are one of the most versatile plastic materials. These can be a found in mattresses, couches, insulation, liquid coatings and paints, tough elastomers such as roller blade wheels, soft flexible foam toys, some elastic fibers, and many other places and applications.
Polyurethanes were invented back in the 1930s by Professor Dr. Otto Bayer (1902-1982). There are various types of polyurethanes, which look and feel very different from each other. Widespread use of polyurethanes was first seen during World War II when they were utilized as a replacement for rubber, which at the time was expensive and hard to obtain. In fact, polyurethane is present in our lives in hundreds of forms, some directly in contact with skin or other fabrics: seats, shoes, coverings, bags, cushions, mattresses, children’s toys, or even prostheses and other surgical materials.
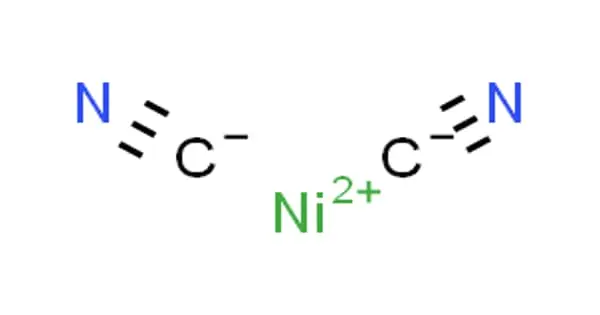
Polyurethane polymers are traditionally and most commonly formed by reacting a di- or triisocyanate with a polyol. Polyurethanes are formed by reacting a polyol with a diisocyanate or a polymeric isocyanate in the presence of suitable catalysts and additives. Since polyurethanes contain two types of monomers, which polymerize one after the other, they are classed as alternating copolymers. Because a variety of diisocyanates and a wide range of polyols can be used to produce polyurethane, a broad spectrum of materials can be produced to meet the needs of specific applications. Both the isocyanates and polyols used to make polyurethanes contain, on average, two or more functional groups per molecule.
Polyurethanes are versatile, modern, and safe. They are used in the manufacture of high-resilience foam seating, rigid foam insulation panels, microcellular foam seals and gaskets, spray foam, durable elastomeric wheels and tires (such as roller coaster, escalator, shopping cart, elevator, and skateboard wheels), automotive suspension bushings, electrical potting compounds, high-performance adhesives, surface coatings, and sealants, synthetic fibers (e.g., Spandex), carpet underlay, hard-plastic parts (e.g., for electronic instruments), condoms, and hoses. They are used in a wide variety of applications to create all manner of consumer and industrial products that play a crucial role in making our lives more convenient, comfortable, and environmentally friendly. It is used in many versatile applications because of its ease of implementation. Despite this advantage, polyurethane also has some disadvantages.
- Durability – Short life is a major disadvantage of polyurethane products. Mattresses made from polyurethane absorb water and gradually disintegrate and lose their quality of support.
- Odor – Polyurethane emits odors and fumes, though they’re not that highly noticeable.
- Health Problems – People who are over exposed to polyurethane experience health problems including allergic reactions, rashes, difficulty breathing, loss of consciousness and even blindness.
Information Source: