Potassium bicarbonate is a solid, crystalline, slightly alkaline, and salty material which is also known as potassium hydrogen carbonate or potassium acid carbonate. This is created by the absorption of carbon dioxide into an aqueous solution of potassium carbonate. It is the inorganic compound with the chemical formula KHCO3. Potassium bicarbonate for the manufacture of potassium carbonate, potassium acetate, potassium arsenite, and other raw materials but also for food, pharmaceuticals, fire extinguishers, antacids, and hair/skin products.

Potassium Bicarbonate (KHCO3) – Powder
Potassium bicarbonate is used as an antacid in medicine. It is registered in the FDA under the section on appropriate, safe, and efficient OTC antacid ingredients. It is produced by treating an aqueous solution of carbonated potassium carbonate:
K2CO3 + CO2 + H2O → 2 KHCO3
Decomposition of the bicarbonate occurs between 100 and 120 °C (212 and 248 °F):
2 KHCO3 → K2CO3 + CO2 + H2O
This reaction is used for the preparation of high purity potassium carbonate. Potassium bicarbonate is formed by reacting with carbon dioxide, then recrystallizing it with potassium carbonate liquid. All equipment is specifically dedicated to potassium bicarbonate, from manufacturing to packaging. It is a commonly used forensic reagent. It is employed as a catalyst in man-made fiber polymerization and olefin dehydrogenation.
Potassium bicarbonate has fungicidal properties and is used for powdery mildew and apple scab protection in organic farming. It has a function as a regulator of food acidity, a raising agent, a buffer, and an agrochemical antifungal. It is a derivative of potassium and an organic derivative. It has hydrogen carbonate in it. It appears as a colorless transparent monoclinic crystal, being water-soluble but alcohol insoluble. Under normal conditions this is stable. Potassium bicarbonate does not contain toxic chemicals and is not classified as either a carcinogen or possible cancer.
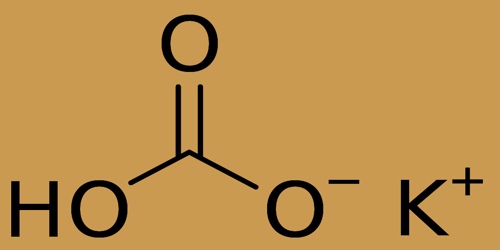
Chemical Structure of Potassium Bicarbonate
Potassium bicarbonate is usually found added to carbonated water to boost taste, and to melt the effect of effervescence. It may also be used as an excipient in drug formulations. An antacid may be a medication accustomed neutralizes gastric acid in a very short timeframe after ingestion and also the effect is soon overcome by meal-stimulated acid secretion. Sodium bicarbonate and potassium bicarbonate are essential components of body tissues that help to control the balance between acid and base of the body. This combination of buffered mineral compounds can help restore the acid/base balance when the body’s own reserves of bicarbonate are depleted because of metabolic acidosis caused by adverse food reactions or other environmental exposures.
Intake of potassium bicarbonate is performed predominantly in the small intestine where approximately 90 percent of potassium is absorbed through passive diffusion. Potassium is excellent for heart health; negative symptoms can occur if a person does not have enough potassium in the body, a disorder known as hypokalaemia. Taking potassium bicarbonate can help to reduce these symptoms. Potassium bicarbonate can also lower pressure and reduce the chance of developing kidney stones. The antacid potential of potassium acid carbonate is attained by increasing the gastrointestinal pH by neutralizing acid.
Potassium bicarbonate is alkaline and leavening agent obtained in colorless prisms or white powder formulations. It is very soluble in 2.8 ml of water with 1 g dis-solving. It releases carbon dioxide when heated and provides leavening in baked goods. Potassium bicarbonate contains no toxic chemicals and is not classified as a carcinogenic substance or as possible cancer. It is also considered safe in pregnancy because the current data don’t suggest a teratogenic potential or any developmental toxicity.
Potassium bicarbonate should be kept in a cool, dry position in a well-closed bottle. Potassium bicarbonate is soluble in the air at normal temperatures but is eventually converted to potassium carbonate when heated to 100–200° C in a dry state, or in solution. In the evolution of carbon dioxide, it reacts in acids and acidic salts. It is used as a fireplace suppression agent (“BC dry chemical”) in some dry chemical fire extinguishers, because of the principal component of the Purple-K dry chemical, and in some applications of condensed aerosol fire suppression.
Information Sources: