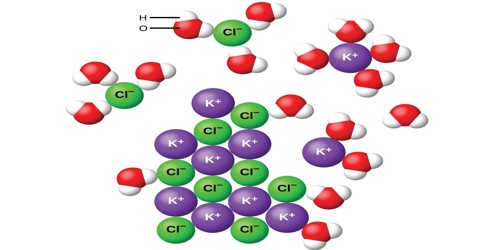
Potassium chloride (also known as potassium salt) is a chemical compound. It is a salt containing a bond between a metal and a halogen. Its chemical formula is KCl. It contains potassium and chloride ions. It is a metal halide composed of potassium and chloride. It is made up of potassium cations and chloride anions in a 1:1 ratio. It is a mineral supplement used to treat or prevent low levels of potassium in the blood.
Properties
It is a colorless crystalline solid. Its molar mass is 74.55 g/mol. It is not reactive. It is similar to sodium chloride. It reacts with silver nitrate to make silver chloride. It reacts with sodium metal when very hot to make potassium metal. The metals are gas because it is so hot. It makes a pink color when heated in a flame. This molecule consists of one potassium cation (K+) and one chloride anion (Cl–).
Physical properties
KCl is an odorless, white crystalline solid, with a density of 1.98 g/mL, a melting point of 770 °C, and a boiling point of 1420 °C.
Occurrence
Potassium chloride is found as a mineral. It occurs naturally as the mineral sylvite. The mineral is called sylvite. It is also found in sylvanite, a mixture of sodium chloride and potassium chloride. It is also present in seawater.
Preparation
KCl is commonly obtained by mining its minerals, followed by extraction. It is taken from the ocean. It can also be processed from the mineral sylvite or sylvanite. It is made when potassium nitrate is reacted with hydrochloric acid, making nitric acid and potassium chloride. It is also extracted from brine (saltwater). It is made when potassium burns in chlorine. It can also be prepared in the laboratory in small scales by reacting potassium hydroxide (KOH) with hydrochloric acid (HCl).
Uses – The main uses of KCl are in electrolytes, pH buffers, and preparation of fertilizers, explosives, potassium metal, and potassium hydroxide.
- Potassium chloride is used to prevent or to treat low blood levels of potassium (hypokalemia).
- It is used to make potassium metal. It is heated very hot until it melts. Sodium metal is reacted with it.
- It can be electrolyzed in a water solution to make potassium hydroxide.
- It is used in food processing.
- It can be used to execute people in a lethal injection.
- It is used instead of table salt sometimes. Normally it is half table salt and half potassium chloride.
- It can be used to extinguish fires.
Safety
Potassium chloride is toxic in very large amounts. When injected into a vein it is much more toxic. High amounts of KCl can affect the cardiac muscles causing heart attacks and even death.